About This Item
- Full TextFull Text(subscription required)
- Pay-Per-View PurchasePay-Per-View
Purchase Options Explain
Share This Item
The AAPG/Datapages Combined Publications Database
GCAGS Transactions
Abstract
Primary Migration - A New Tack on an Old Theme
Jack I. Simmons (1)
ABSTRACT
Hydrocarbon droplets small enough to migrate through source rock pores contain too few molecules to follow fluid laws. Further, their solubility in water is greater than that of larger droplets. This should form a concentration gradient preferentially diffusing hydrocarbons toward larger pore spaces. Once emplaced, possibly as pools, their large size and corresponding low solubilities tend to stabilize them against further solution movement. A sample calculation of such diffusion drive can account for the size of observed pools in the Gulf Coast.
DISCUSSION
Primary migration of hydrocarbons is their movement through the pores of the source rock into rocks of sufficient pore size to serve as a carrier bed to a reservoir. The size ranges of these pores are described by Rieke and Chillingarian (1974), who note that these pores in argillaceous rocks are quite small, perhaps 10-5 to 10-7 cm in size. The size of a droplet that can be contained within these pores must be of that size or smaller, and will contain relatively few hydrocarbon molecules, perhaps a thousand or so. As an example, a spherical droplet of 10-6 cm in diameter would contain less than 2,000 molecules of the common paraffin C20H42.. Fluid properties such as viscosity, capillarity and surface tension should not rigidly apply to these small numbers of molecules. Also, the fluid laws governing single-phase flow would be hard to evaluate.
The oil droplets in source rock pores are normally a small percent of the connate fluids. They must exist as a very finely-divided emulsion in the connate water, stabilized from coalescing into larger sizes by the intervening rock-matrix particles. An important factor to consider in the physical characteristics of such hydrocarbon size ranges is the high solubility these small droplets would show in the connate water, much larger than a solubility measured on a large volume of the same hydrocarbon in the same water. Low hydrocarbon-solubilities have been measured by Price (1976), and have been a deterrent in postulating effective diffusion migration and/or the expulsion of hydrocarbons with dewatering by compaction. An analogue to the Kelvin equation (Sherman, 1968), states that the solubilities of emulsion droplets are inversely related to the size of the droplets, or small droplets are more soluble than large droplets.
A plot of this equation for the paraffin C20H42 at 200° is presented in Figure 1. The solubilities of small droplets that could be contained within source rock pores (S1) can be many times the solubility of reservoir droplets (S2).
- Hydrocarbons formed in the fine-grained organic material of a source rock will be constrained to be of sizes smaller than their confining pores.
- The solubilities of these small droplets will be large, creating aureoles of high dissolved-hydrocarbon concentration about themselves. These high-concentration aureoles will expand by diffusion through the source rock with time.
- When large pore spaces are available and suitable nuclei (larger droplets) are present, the larger droplet will act as a sink to lower the dissolved-hydrocarbon concentration about itself. A concentration gradient is formed between the smaller droplet and the larger, driving hydrocarbons by diffusion into the larger droplet size ranges. In order for this drive to work efficiently, it would appear necessary to have good nucleation potential in near-juxtaposition to reservoir or carrier beds.
FIGURE 1. A plot of the Kelvin analogue for the solubility ratio [S1]/[S2] of C20H42 oil droplets as a function of smallest droplets radius  1.
1.
End_Page 535------------------------
The rate at which such diffusive transfer of hydrocarbons will take place can be calculated from Fick's first law as described by Jost (1952).
Q = -- D [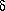 C]/[
C]/[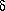 x]
x]
Where Q = the mass flow per unit area per unit time, D is the constant of diffusivity for the system and [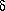 C]/[
C]/[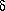 x] is the concentration gradient of solute hydrocarbon along the travel path x.
x] is the concentration gradient of solute hydrocarbon along the travel path x.
The mean distance a hydrocarbon molecule can travel in any time t was found by Einstein (1906) to be dependent upon the diffusivity constant and time, or:
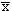 2 = 2Dt
2 = 2Dt
where 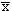 2 is the mean square of the displacement.
2 is the mean square of the displacement.
Both the rates of diffusive flow and distances that can be covered appear small due to the small coefficient D (about 10-5 cm2/sec after Bonoli and Witherspoon, 1968),but the large time-span for these geologic processes is usually great enough to permit large volumes of movement over great distances. As an example, plots of the mean distance traveled by molecules of C20H42 and CH4 (methane) are presented in Figure 2. The distances traveled in time by these molecules are shown for their travel in water, and for one-fourth of that distance to allow for the tortuosity of source-rock pores. This tortuosity is relatively unknown, but a sample calculation for platy matrix materials at various plate length/thickness ratios is presented in Figure 3. A tortuosity of less than five is suggested.
Let's calculate what amounts of a C20H42 molecule might move into one sand through Kelvin diffusion flow. The example could encompass a large Gulf Coast structure with an area of uplift (A) = 15 x 15 miles or 1.17 x 1013 cm2 for a migration time span per million years of 3.1 x 1013 sec. The diffusive rate of flow of hydrocarbon Q is approximately equal to -- D [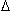 C]/[
C]/[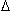 x] where [
x] where [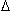 C]/[
C]/[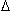 x] for a 100 m shale in contact with a carrier bed is about 5 x 10-10 gm/cm2.
x] for a 100 m shale in contact with a carrier bed is about 5 x 10-10 gm/cm2. 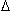 C is estimated from the work of Bonoli (1968) and Price (1976), combined with the data from Figure 2 for a reservoir/source relationship, while
C is estimated from the work of Bonoli (1968) and Price (1976), combined with the data from Figure 2 for a reservoir/source relationship, while 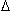 x, the mean of how far a molecule can move, is compatible with Figure 2 and with a Gulf Coast interbedded source/reservoir model. D can be estimated from the Wilke-Chang
x, the mean of how far a molecule can move, is compatible with Figure 2 and with a Gulf Coast interbedded source/reservoir model. D can be estimated from the Wilke-Chang
FIGURE 2. The mean distance a molecule can move in water with time.
FIGURE 3. Tortuosity vs. porosity of shale source rock, planar & parallel shale platelets; maximized shale path length.
End_Page 536------------------------
equation using the published data of Bonoli and Witherspoon (1968) and Perry et al., (1963) to be 1.7 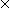 10-5 cm2 sec-1 for the C20H42 molecule.
10-5 cm2 sec-1 for the C20H42 molecule.
The total flow into one carrier/reservoir bed per time span of one million years is approximately the flow rate per unit area times the surface area of the reservoir times time, or 61.6 x 1011 grams of hydrocarbon may be emplaced in the reservoir/carrier. This approximates 38 x 106 bbl. oil per sand (in place). With maximum reservoir/source interfingering, fields of hundreds of millions of recoverable barrels could be created in one million years. These values seem compatible with observed accumulations in the Gulf Coast.
Hydrocarbons are soluble in water, and if generated before or during compaction, must be moved into carrier bids with expelled water, and perhaps into reservoirs. The effectiveness of this movement combined with the increased Kelvin solubility in source rocks can be estimated. Assuming 10 percent water expelled in the generative window -- and 30 x 10-6 ppm hydrocarbons carried (Kelvin -- this paper), per 15 x 15 mi structure and one mile of generative section, 22.5 miles of water are lost (or 576 x 109 bbl), carrying with it 17 million barrels of C20H42. This amount (calculated per structure) is about half that calculated per sand by the Kelvin diffusion calculation, and seems to be an order of magnitude under the diffusion accumulation expected in a well-interfingered, reservoir-source sequence.
The Kelvin low solubility for large oil pools indicates that the reservoir not only accumulates hydrocarbons but tends to hold them through long time periods. Once the primary migration is in progress and the cap rock is saturated with hydrocarbon, little diffusive migration through the cap can occur, except that caused by long-range gradients of low efficiency such as thermal gradient diffusion.
In conclusion, this study suggests that diffusion primary migration after the laws of Fick, Einstein and Kelvin is likely to be a large (or dominating) factor for at least some source -- reservoir conditions.
Pay-Per-View Purchase Options
The article is available through a document delivery service. Explain these Purchase Options.
| Watermarked PDF Document: $14 | |
| Open PDF Document: $24 |
