About This Item
- Full TextFull Text(subscription required)
- Pay-Per-View PurchasePay-Per-View
Purchase Options Explain
Share This Item
The AAPG/Datapages Combined Publications Database
GCAGS Transactions
Abstract
Petrography and Geochemistry of the Matagorda Island 519 Field, Offshore Texas
Bob Klein (1), Lori Hathon (2), Charles Brewster (3)
ABSTRACT
Lower Miocene sandstones at the Matagorda Island 519 field (Figs. 1 and 2) are productive in an offstructure, geopressured setting. To further understand the dynamics of the field's origin, extensive petrology and geochemistry of field cores, cuttings, and fluids were performed. The results were used to support an exploration model that identifies geopressured exploration targets that may have a similar genetic origin.
At Matagorda Island 519 field, geopressured deltaic sandstones are productive from depths of 13,500 to 16,000 ft. Reservoir sandstones display excellent reservoir quality, with porosities between 20% and 25%, and permeabilities ranging from 0.02 to 300 md. The good reservoir quality is the result of the generation of secondary porosity. The following sequence of diagenetic events is inferred from petrographic observations: 1) mechanical compaction, 2) local precipitation of chlorite grain coats on detrital grains, 3) calcite cementation, 4) partial to complete dissolution of detrital grains and calcite cement, and, 5) local recementation by ankerite, pyrite, and polycrystalline quartz. Using observed intergranular volumes of 25 to 33 percent, together with burial history analysis and sandstone compaction models, it is determined that calcite cement was emplaced early and shallow (at depths less than 8000 ft).
Detailed conductivity log correlations led to the identification of four banded conductivity compartments that are also individual geopressured cells (Figs. 3 and 4). Shale cuttings samples were obtained every several hundred feet from the well bore and were analyzed by x-ray fluorescence (XRF) techniques to determine their trace element chemical composition. (These shale-siltsone samples were hand selected to avoid contamination by sand and drilling additives.) The rationale was that mudrocks should not be as prone as sandstones to display chemical composition variations due to changes in provenance. Thus the mudrocks may preserve the diagenetic effects of geopressure cell formation and water-rock interactions imposed by basin-scale fluid flow. The trace element composition data were normalized relative to TiO2, which is thought to be relatively immobile in the subsurface.
The normalized trace element data tracked the conductivity/geopressured cells distinctly. The data suggest the export of Fe203, SiO2, MgO, and CaO from mudrocks in deep geopressure, and enrichment of these components in mudrocks capping the uppermost geopressured cell. In Figure 5, the depletion from compartment D and the enrichment in compartment A can be seen. For K2O (Fig. 6), the trend is reversed: each deeper pressure cell becomes more enriched in K2O as it is incorporated into illite clay structure. The data show a vertical transport of some geochemically mobile elements over distances of 5000 to 6000 ft. This concept is similar to the concept of Land, et al. (1997) of open chemical diagenesis within geopressured rocks.
Additional XRD and fluid inclusion chemical analyses were performed on splits of the same samples analyzed for XRF. These results are summarized in Figure 7: smectite contents of about 75 percent are contained in mudrocks to a depth of 10,000 ft; compartment A records the start of the smectite to illite transformation. (This transformation conicides not only with the occurence of tightly calcite-cemented, high resistivity sandstones, but also with increased CO2 content in the rocks' fluid inclusions.) Within compartment A, most of the smectite to illite transition is accomplished, as smectite content is reduced from 75 percent to 30 percent in the mudrocks. Within compartments B through D only about 5 percent illite is gained. This may suggest an irreducible quantity of smectite, dependent on potassium availability.
Rock samples were collected from three cores in the field and were geochemically analyzed for Sr, O and C isotopic values (Table 1). Calcite cement has 87/86 Sr values of 0.70805 to 0.71011. These values eliminate coeval marine seawater (approximately 18 mya) and marine carbonate (0.7084 to 0.7086) as the only sources of strontium. The most radiogeneic strontium values are found updip, in the most fault-proximal well, the OCS 5169#1 (Fig. 8). Radiogenic values at this location are attributed to late-stage, ankerite-precipitating fluid which is inferred to have migrated during the latter stages of field charging by hydrocarbons. A distinct, linear covariation trend between 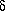 18O and
18O and 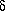 13C values is observed. This trend is interpreted to represent a mixing between end member detrital calcite and authigenic calcite phases (Figs. 9 and 10). The end member authigenic calcite is estimated to have
13C values is observed. This trend is interpreted to represent a mixing between end member detrital calcite and authigenic calcite phases (Figs. 9 and 10). The end member authigenic calcite is estimated to have 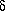 18O~ -8 to -10 per mil, PDB. Based on the inferred
18O~ -8 to -10 per mil, PDB. Based on the inferred 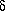 18O of the authigenic calcite, and using typical
18O of the authigenic calcite, and using typical 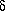 18O values for the subsurface in the Gulf of Mexico (GOM) (4.9 to 5.6 per mil, PDB) we estimate calcite cementation temperatures to be between 205° F and 256° F (Fig. 11). These temperatures are inconsistant with shallow calcite precipitation, as the local geothermal gradient shows a temperature of about 180°F at 8000 ft. However, the high temperature of calcite cementation suggested by the oxygen isotopes can be reconciled with the shallow depths of calcite cementation if hydrothermal fluids migrating up the expansion fault system were the cementing fluids.
18O values for the subsurface in the Gulf of Mexico (GOM) (4.9 to 5.6 per mil, PDB) we estimate calcite cementation temperatures to be between 205° F and 256° F (Fig. 11). These temperatures are inconsistant with shallow calcite precipitation, as the local geothermal gradient shows a temperature of about 180°F at 8000 ft. However, the high temperature of calcite cementation suggested by the oxygen isotopes can be reconciled with the shallow depths of calcite cementation if hydrothermal fluids migrating up the expansion fault system were the cementing fluids.
Well-head gas samples were collected to identify the sulfur isotopic signature of the H2S component of the hydrocarbons. Sulfur isotope compositions of +1.9 to +3.9 (per mil, CDT) were observed. The H2S component of the gas could have evolved through either biogenic or thermochemical sulfate reduction pathways, however, the source was probably thermochemical: there are no known bacteria that can survive in the 330° F environment observed in the perforated interval, and the observed carbon isotope compositions (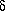 13C~-40 to -43 per mil, PDB) of the methane is characteristic of thermogenic rather than biogenic origin. The following scenario is consistent with a deep-basin source of H2S: TSR of sulfate from the Eagle Mills evaporate (
13C~-40 to -43 per mil, PDB) of the methane is characteristic of thermogenic rather than biogenic origin. The following scenario is consistent with a deep-basin source of H2S: TSR of sulfate from the Eagle Mills evaporate (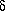 34S ~12 per mil) at temperatures between 330° F and 360° F would produce hydrogen sulfide with a range of
34S ~12 per mil) at temperatures between 330° F and 360° F would produce hydrogen sulfide with a range of 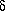 34S values between -3 and +5 per mil. The range of observed values are well within these limits (Fig. 12). If the source of the sulfate is from the evaporite units of the deeply
34S values between -3 and +5 per mil. The range of observed values are well within these limits (Fig. 12). If the source of the sulfate is from the evaporite units of the deeply
End_Page 547------------------------
Figure 1. Location of Matagorda Island 519 field, offshore Texas.
End_Page 548------------------------
buried Mesozoic units, then a very significant volume of fluids have passed along the same migration pathway of the hydrocarbons.
Our examination of the Matagorda 519 field suggests that the gas-condensate accumulation results from episodic fluid flow events and a complex sequence of diagenetic events. These diagenetic events form the pressure cells observed and are thought to arise through the sequence of events shown on Figure 13. Geochemical data are consistent with the hypothesis that deep, basinal fluids are responsible for the initial calcite cementation of the reservoir unit and its inferred subsequent dissolution. The late stage mineral zonation of ankerite, pyrite and polycrystalline quartz in the updip, and most fault proximal position are interpreted to be a direct hydrocarbon indicator for this type of field. Sr isotope data suggest a very high degree of water-rock interaction, which is another criterion by which these fields may be recognized. The late stage mineral assemblage effectively acts as pore-throat seal for the hydrocarbons reservoired downdip. The recognition of these diagenetic mineral assemblages and isotopic chemical signatures may have significant exploration implications in this gas-condensate prolific trend.
Pay-Per-View Purchase Options
The article is available through a document delivery service. Explain these Purchase Options.
| Watermarked PDF Document: $14 | |
| Open PDF Document: $24 |
