About This Item
- Full TextFull Text(subscription required)
- Pay-Per-View PurchasePay-Per-View
Purchase Options Explain
Share This Item
The AAPG/Datapages Combined Publications Database
AAPG Special Volumes
Abstract
Pub. Id:
First Page:
Last Page:
Book Title:
Article/Chapter:
Subject Group:
Spec. Pub. Type:
Pub. Year:
Author(s):
Abstract:
Methanic diagenesis commonly dominates pore-water chemistry in organic carbon-rich sediments from depths of tens of centimeters to 1000 m or more, and is coincident with the depths of principal sediment dewatering. As a result, methanic diagenesis in organic carbon-rich mudstones may control early diagenesis in adjacent sand and sandstone and can result in the accumulation of economic quantities of biogenic methane. Chemical aspects of methanic diagenesis in ancient marine sediments can be reconstructed by analogy with processes in modern diagenetic environments, on the basis of the mineralogy, texture, and isotopic composition of concretionary carbonate cements and other related authigenic minerals.
This sort of diagenetic reconstruction is well illustrated by means of examples from the Upper Cretaceous Gammon Shale from southeastern Montana. Bioturbated mudstones of the Gammon accumulated in oxic, open marine waters, but dissolved oxygen was probably depleted from pore waters a few tens of centimeters beneath the sediment/water interface. Sulfate reduction took place beneath this depth and was most important in a zone of mixing at the base of bioturbation, where isotopically light (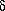
Interpretation of methanic diagenesis in the Gammon Shale illustrates only part of a single diagenetic pathway for one type of organic carbon-rich mudrock. And yet, the implications are clear: Early diagenesis of muds is dominated by processes involving organic matter and by the products of organic matter decomposition. Because of the economic significance of organic carbon-rich mudrocks as source beds for hydrocarbons and because their diagenesis probably controls mineral precipitation and dissolution in many reservoir rocks, it is of the utmost importance that diagenesis in ancient mudstones be understood.
Pay-Per-View Purchase Options
The article is available through a document delivery service. Explain these Purchase Options.
| Watermarked PDF Document: $14 | |
| Open PDF Document: $24 |
