The AAPG/Datapages Combined Publications Database
AAPG Bulletin
Full Text
![]() Click to view page images in PDF format.
Click to view page images in PDF format.
AAPG Bulletin, V.
DOI: 10.1306/08291717097
Understanding and distinguishing reflectance measurements of solid bitumen and vitrinite using hydrous pyrolysis: Implications to petroleum assessment
Paul C. Hackley,1 and Michael Lewan2
1US Geological Survey, MS 956 National Center, 12201 Sunrise Valley Drive, Reston, Virginia 20192; Department of Chemistry and Biochemistry, George Mason University, MS 3E2, Fairfax, Virginia 22030; [email protected]
2US Geological Survey, MS 977 Denver Federal Center, Box 25046, Denver, Colorado 80225; [email protected]
ABSTRACT
Solid bitumen is a common organic component of thermally mature shales and typically is identified by embayment against euhedral mineral terminations and by groundmass textures. However, because these textures are not always present, solid bitumen can be easily misidentified as vitrinite. Hydrous-pyrolysis experiments (72 hr, 300°C–360°C) on shale and coal samples show that solid-bitumen reflectance (BRo) in shales is less responsive to thermal stress than vitrinite reflectance (Ro) in coal. This effect is most pronounced at lower experimental temperatures (300°C–320°C), whereas reflectance changes are more similar at higher temperatures (340°C–360°C). Neither a “vitrinite-like” maceral nor “suppressed vitrinite” was identified or measured in our sample set; instead, the experiments show that solid bitumen matures slower than vitrinite. The data may explain some reports of “Ro suppression,” particularly at lower thermal maturity (Ro ≤ 1.0%), as a simple case of solid bitumen being mistaken for vitrinite. Further, the experimental results confirm previous empirical observations that Ro and BRo are more similar at higher maturities (Ro > 1.0%). It is suggested that Ro suppression, commonly reported from upper Paleozoic marine shales of early to midoil window maturity, is a misnomer. This observation has important implications to petroleum exploration models and resource assessment, because it may change interpretations for the timing and spatial locations of kerogen maturation and petroleum generation.
INTRODUCTION
Vitrinite is a kerogen maceral, an insoluble organic residue from woody precursor materials in sedimentary rocks, including coal (Taylor et al., 1998). The reflectance of light incident upon vitrinite under oil immersion (Ro) in polished rock samples increases systematically with increasing thermal maturity, usually related to maximum depth of burial (Dow, 1977). This change occurs because of chemical reactions including increased aromatization and condensation in response to higher temperatures and the molecular rearrangement of vitrinite into planar structures corresponding to the anisotropic stress field encountered at depth in the Earth’s crust (McCartney and Teichmüller, 1972; Levine and Davis, 1984). Because of this predictable change, measurement of Ro is considered one of the more robust of many techniques available for determination of thermal maturity for petroleum exploration and basin analysis (Mukhopadhyay and Dow, 1994; Suárez-Ruiz et al., 2012; Hackley et al., 2015; Hackley and Cardott, 2016).
Pre-oil solid bitumen occurs in sedimentary rocks as an early product of oil-prone kerogen conversion to petroleum (mainly from algal and bacterial biomass precursors, Tissot and Welte, 1984; Curiale, 1986; Hunt, 1996). Because of similar chemical reactions that occur in vitrinite, the reflectance of solid bitumen under oil immersion (BRo) also increases with increasing thermal maturity, and BRo is sometimes  used
used as a thermal-maturity parameter in addition to or in place of Ro (Riediger, 1993; Hackley, 2012; Petersen et al., 2013; Valentine et al., 2014). At low thermal maturity (Ro < 1.0%), the reflectance of solid bitumen typically is lower than the reflectance of co-occurring vitrinite, that is, Ro ≥ BRo when Ro < 1.0%, as observed by empirical studies (Robert, 1988; Landis and Castaño, 1995; Schoenherr et al., 2007; Ferreiro Mählmann and Frey, 2012; Ferreiro Mählmann and Le Bayon, 2016; Wei et al., 2016). Because solid bitumen is a petroleum product generated from conversion of oil-prone organic matter or from alteration of liquid oil (termed post-oil solid bitumen) (Cardott et al., 2015; Hackley and Cardott, 2016), it contains in its molecular structure some proportion of an oil-like saturated n-alkane component (Curiale, 1986), which theoretically reduces its overall aromaticity relative to vitrinite and therefore lowers its relative reflectance (Wei et al., 2016). Apparent cleavage of aliphatic parts of the solid-bitumen molecule with increasing thermal maturity results in increased aromaticity, and solid bitumen and vitrinite typically have overlapping reflectance values when Ro is greater than 1.0% (Landis and Castaño, 1995). Although we consider solid bitumen identified in this study to be a product of petroleum formation, macerals similar in appearance to solid bitumen (“vitrinite-like” macerals) may be syndepositional and not a product of petroleum formation (Houseknecht and Matthews, 1985; Buchardt and Lewan, 1990; Xiao et al., 2000; Schmidt et al., 2015).
as a thermal-maturity parameter in addition to or in place of Ro (Riediger, 1993; Hackley, 2012; Petersen et al., 2013; Valentine et al., 2014). At low thermal maturity (Ro < 1.0%), the reflectance of solid bitumen typically is lower than the reflectance of co-occurring vitrinite, that is, Ro ≥ BRo when Ro < 1.0%, as observed by empirical studies (Robert, 1988; Landis and Castaño, 1995; Schoenherr et al., 2007; Ferreiro Mählmann and Frey, 2012; Ferreiro Mählmann and Le Bayon, 2016; Wei et al., 2016). Because solid bitumen is a petroleum product generated from conversion of oil-prone organic matter or from alteration of liquid oil (termed post-oil solid bitumen) (Cardott et al., 2015; Hackley and Cardott, 2016), it contains in its molecular structure some proportion of an oil-like saturated n-alkane component (Curiale, 1986), which theoretically reduces its overall aromaticity relative to vitrinite and therefore lowers its relative reflectance (Wei et al., 2016). Apparent cleavage of aliphatic parts of the solid-bitumen molecule with increasing thermal maturity results in increased aromaticity, and solid bitumen and vitrinite typically have overlapping reflectance values when Ro is greater than 1.0% (Landis and Castaño, 1995). Although we consider solid bitumen identified in this study to be a product of petroleum formation, macerals similar in appearance to solid bitumen (“vitrinite-like” macerals) may be syndepositional and not a product of petroleum formation (Houseknecht and Matthews, 1985; Buchardt and Lewan, 1990; Xiao et al., 2000; Schmidt et al., 2015).
Geologists have long noted instances where Ro appears to be suppressed (low) relative to expected values, and several mechanisms have been invoked to explain “vitrinite-reflectance suppression” based on empirical studies. Some workers have postulated that suppressed vitrinite has atypical maturation kinetics because of enrichment in hydrogen or sulfur (Hutton and Cook, 1980; Price and Barker, 1985; Hao and Chen, 1992; Ujiié et al., 2004; Barker et al., 2007). Others have hypothesized that vitrinite maturation kinetics are retarded via overpressured conditions caused by the impermeability of fine-grained sediments, which prevent forward reaction (McTavish, 1978, 1998; Hao et al., 1995, 2007; Carr, 2000; Li et al., 2004).
Vitrinite and solid bitumen typically occur as structureless gray “blebs” of low relief in reflected white light when dispersed in sedimentary rocks such as shale and can be difficult to distinguish petrographically. Solid bitumen is a mobile, viscous material (Curiale, 1986) that conforms to voids and pores in rock matrices, and organic petrographers differentiate solid bitumen from vitrinite based on the presence of unambiguous void filling (embayment by euhedral mineral terminations) or groundmass textures. However, solid bitumen that lacks these textures can be indistinguishable from vitrinite. Furthermore, precursors to vitrinite undergo a gelification process during early diagenesis, and some workers have suggested that malleable proto-vitrinite gels can readily deform around mineral grains or fill pores when dispersed in sediments during early compaction (Chaffee et al., 1984; Russell, 1984; Stout and Spackman, 1987), possibly resulting in textures that resemble solid bitumen. Because of the visual similarities between solid bitumen and vitrinite, the misidentification of solid bitumen as vitrinite has been postulated to result in reports of Ro suppression at lower thermal maturities (Hackley et al., 2013; Ryder et al., 2013). Furthermore, because misidentification of solid bitumen as vitrinite can affect the predicted spatial distribution of thermally mature self-sourced shale reservoirs or thermal-history reconstructions, this issue can adversely impact petroleum assessments. In particular, the common occurrence of solid bitumen in the petroleum-producing marine-shale reservoirs of North America (Hackley and Cardott, 2016) demands that the distinctions between solid bitumen and vitrinite be precisely documented to improve estimates of thermal-maturation profiles and the timing of petroleum generation.
To determine if reports of Ro suppression may be linked to misidentification of solid bitumen as vitrinite, we tested the response of vitrinite in coals and pre-oil solid bitumen in shales to thermal stress. A suite of samples was artificially matured via hydrous-pyrolysis laboratory experiments at high temperatures (300°C–360°C). These experiments documented differences in the maturation kinetics of the two types of organic matter. The results suggest that the reflectance of solid bitumen is lower than that of co-occurring vitrinite because of differences in a priori composition that cause differences in maturation kinetics. This exposes a  potential
potential reason behind reports of Ro suppression, particularly in early mature upper Paleozoic marine shales, as the simple misidentification of solid bitumen as vitrinite. Better understanding of the rationale behind reports of Ro suppression such as described herein may decrease uncertainties in determination of thermal history and allow more accurate prediction of the locations of generation, migration, and accumulation of petroleum resources, ultimately improving hydrocarbon (HC) exploration.
reason behind reports of Ro suppression, particularly in early mature upper Paleozoic marine shales, as the simple misidentification of solid bitumen as vitrinite. Better understanding of the rationale behind reports of Ro suppression such as described herein may decrease uncertainties in determination of thermal history and allow more accurate prediction of the locations of generation, migration, and accumulation of petroleum resources, ultimately improving hydrocarbon (HC) exploration.
METHODS
Materials
For hydrous-pyrolysis experiments, 8 coal samples and 14 shale samples (Table 1) were  used
used as starting materials. Samples included a wide variety of shale and coal, all with abundant organic matter and of low thermal maturity. Samples were from diverse depositional environments (marine, lacustrine, and terrestrial coal measures) and ranged in geologic age from Cambrian–Ordovician to Miocene.
as starting materials. Samples included a wide variety of shale and coal, all with abundant organic matter and of low thermal maturity. Samples were from diverse depositional environments (marine, lacustrine, and terrestrial coal measures) and ranged in geologic age from Cambrian–Ordovician to Miocene.
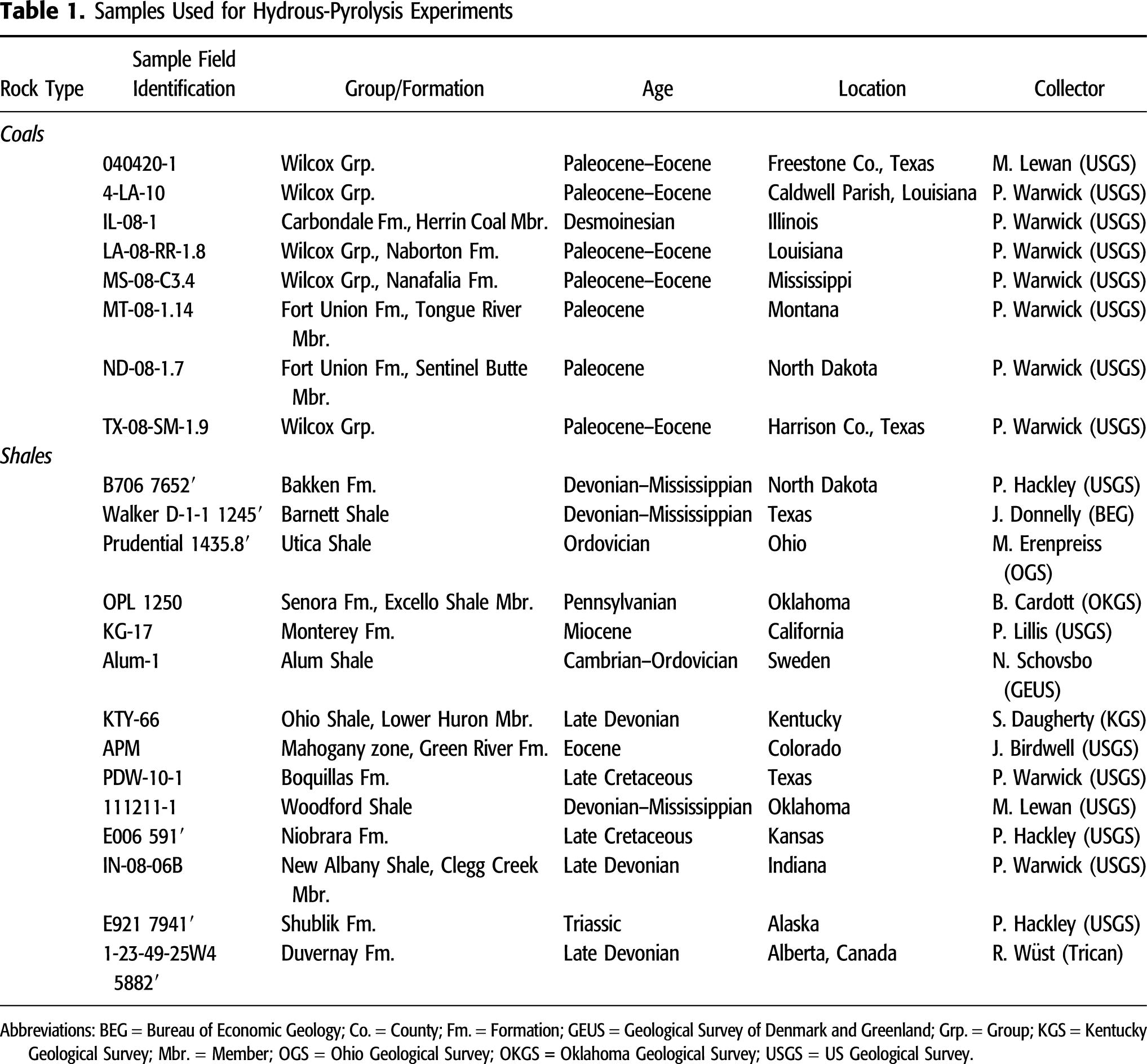
Hydrous Pyrolysis
The hydrous-pyrolysis method followed the approach of Lewan (1993b, 1997), wherein crushed-rock samples (2–4 g, 1–3 mm top size) were loaded into reactors and covered with deionized water (Figure 1). Sufficient water was added to keep the sample covered and in constant contact with water at the temperature of each experiment. To avoid catastrophic rupture of the reactors during experiments resulting from liquid expansion, the water and rock amounts  used
used were calculated based on reactor internal volumes, rock densities, and steam tables as described by Lewan (1993b). The stainless steel (SS-316) SwageLok minireactor vessels (25–35-ml internal volume) were assembled from 1.5-in. (3.81-cm) tubing caps and plugs, sealing with approximately 270 ft-lb (82 m-lb) torque using Fel-Pro C5-A copper-based antiseize thread lubricant. No attempt was made to evacuate the reactor head space. Once sealed, reactors were weighed to ±0.01 g, placed into gas-chromatograph ovens, and isothermally heated for 72 hr at temperatures of 300°C, 320°C, 340°C, 350°C, and 360°C. The time needed for ovens to reach experimental temperature (generally 5–10 min) was not included in 72-hr experiment durations. At the conclusion of heating, the reactors were reweighed to monitor against leakage then opened and reweighed to estimate outgassing. Pyrolyzed rock residues were removed and rinsed in acetone to remove generated bitumen from rock surfaces. Acetone was
were calculated based on reactor internal volumes, rock densities, and steam tables as described by Lewan (1993b). The stainless steel (SS-316) SwageLok minireactor vessels (25–35-ml internal volume) were assembled from 1.5-in. (3.81-cm) tubing caps and plugs, sealing with approximately 270 ft-lb (82 m-lb) torque using Fel-Pro C5-A copper-based antiseize thread lubricant. No attempt was made to evacuate the reactor head space. Once sealed, reactors were weighed to ±0.01 g, placed into gas-chromatograph ovens, and isothermally heated for 72 hr at temperatures of 300°C, 320°C, 340°C, 350°C, and 360°C. The time needed for ovens to reach experimental temperature (generally 5–10 min) was not included in 72-hr experiment durations. At the conclusion of heating, the reactors were reweighed to monitor against leakage then opened and reweighed to estimate outgassing. Pyrolyzed rock residues were removed and rinsed in acetone to remove generated bitumen from rock surfaces. Acetone was  used
used as solvent because of its effectiveness at removal of polar bitumen. Residues were vacuum dried overnight prior to preparation for petrographic analysis.
as solvent because of its effectiveness at removal of polar bitumen. Residues were vacuum dried overnight prior to preparation for petrographic analysis.
 Figure 1. Photographs to illustrate the hydrous-pyrolysis equipment and analytical approaches employed. (A) (1) Base, (2) top, and (3) assembled reactor with crushed-rock sample in jar and deionized water. The types of analyses are listed for the sample starting materials. (B) Reactors in gas-chromatograph oven. (C) Post-experiment opened reactor showing oil generated from kerogen floating on water. (D) Post-hydrous-pyrolysis rock residue and types of analyses.
Figure 1. Photographs to illustrate the hydrous-pyrolysis equipment and analytical approaches employed. (A) (1) Base, (2) top, and (3) assembled reactor with crushed-rock sample in jar and deionized water. The types of analyses are listed for the sample starting materials. (B) Reactors in gas-chromatograph oven. (C) Post-experiment opened reactor showing oil generated from kerogen floating on water. (D) Post-hydrous-pyrolysis rock residue and types of analyses.
Analysis of Materials
The starting materials and experimental products were analyzed for multiple thermal-maturity proxies including Ro (of coals) and BRo (of shales) and also via Rock-Eval II programmed pyrolysis. Starting materials and pyrolysis residues were prepared for petrographic analyses according to ASTM D2797 (ASTM International, 2015a), wherein the rock particles are mounted in a thermoset plastic briquette then ground and polished with successively finer abrasives until a 0.05-μm finishing stage. Petrographic analyses were performed at the US Geological Survey (USGS) in Reston using ASTM D2798 for Ro of coal (ASTM International, 2015b) and ASTM D7708 (ASTM International, 2015c) for BRo in shale. In brief, incident white light is reflected from selected vitrinite and solid-bitumen particles positioned under the microscope crosshairs at 500× magnification, measured at a detector, and compared with measured light reflected from a calibration standard. At least 20 individual measurements of BRo were determined for each shale sample, and 50–100 measurements were determined for Ro in coal. Standard-deviation values ranged from 0.03 to 0.06 for BRo and Ro in the starting materials. Standard-deviation values generally were 0.05–0.06 for high-temperature coal residues and higher for solid bitumen (generally 0.10–0.15) in high-temperature shale residues. Sample briquettes were imaged under oil immersion on a Zeiss AxioImager microscope in white and blue incident light. A Leica DM4000 microscope equipped with light-emitting-diode illumination and monochrome camera detection was  used
used for reflectance analysis with the computer program DISKUS-FOSSIL by Hilgers Technisches Buero. A yttrium-aluminum-garnet calibration standard (0.908% Ro) from Klein and Becker was
for reflectance analysis with the computer program DISKUS-FOSSIL by Hilgers Technisches Buero. A yttrium-aluminum-garnet calibration standard (0.908% Ro) from Klein and Becker was  used
used .
.
Because the primary objective of this study was to differentiate post-experiment differences in Ro versus BRo, extreme caution was applied to selection of objects for measurement in shales where both macerals can be present (Devonian and younger only). Therefore, solid-bitumen measurements were only determined on gray reflecting macerals that exhibited void filling (embayment) or groundmass textures (Figure 2), similar to petrographic descriptions of solid bitumen from previous workers (Lewan, 1987). To avoid  potential
potential misidentification as solid bitumen of vitrinite that had preserved gelification textures from early diagenesis, discrete solitary particles were avoided, and locations for measurement were sought that showed continuity with groundmass solid bitumen or lamellae. Further, BRo measurements were determined only on native pre-oil solid bitumens that were interpreted to be present in the starting materials and that evolved in reflectance during the experiments. Solid bitumens that were not present in the original samples but instead were generated from oil-prone kerogens during the hydrous-pyrolysis experiments (restricted to the Eocene Green River Mahogany-zone oil-shale sample) were measured but recognized as unrelated to the native pre-oil solid bitumens present in the original sample. The petrographic distinction of native versus newly generated solid bitumen was based on texture; native solid bitumens in the Mahogany-zone sample occurred as thin (5–10 μm) lamellae parallel to bedding, whereas newly generated solid bitumen in pyrolysis residues replaced amorphous kerogen with a wispy texture that was dispersed in the rock matrix. The term “solid bitumen” as
misidentification as solid bitumen of vitrinite that had preserved gelification textures from early diagenesis, discrete solitary particles were avoided, and locations for measurement were sought that showed continuity with groundmass solid bitumen or lamellae. Further, BRo measurements were determined only on native pre-oil solid bitumens that were interpreted to be present in the starting materials and that evolved in reflectance during the experiments. Solid bitumens that were not present in the original samples but instead were generated from oil-prone kerogens during the hydrous-pyrolysis experiments (restricted to the Eocene Green River Mahogany-zone oil-shale sample) were measured but recognized as unrelated to the native pre-oil solid bitumens present in the original sample. The petrographic distinction of native versus newly generated solid bitumen was based on texture; native solid bitumens in the Mahogany-zone sample occurred as thin (5–10 μm) lamellae parallel to bedding, whereas newly generated solid bitumen in pyrolysis residues replaced amorphous kerogen with a wispy texture that was dispersed in the rock matrix. The term “solid bitumen” as  used
used in this study applies to all solid bitumens observed, regardless of their relative solubility, ranging from the pre-oil native solid bitumens in the starting materials to the postmature solid bitumens present in the residues of the 360°C hydrous-pyrolysis experiments.
in this study applies to all solid bitumens observed, regardless of their relative solubility, ranging from the pre-oil native solid bitumens in the starting materials to the postmature solid bitumens present in the residues of the 360°C hydrous-pyrolysis experiments.
 Figure 2. Photomicrographs of example textures
Figure 2. Photomicrographs of example textures  used
used to identify solid bitumen in shale sample starting materials and hydrous-pyrolysis residues. (A) Solid bitumen filling chambers of Globigerina foraminifera in Boquillas Formation (Eagle Ford Formation equivalent) marl in 320°C hydrous-pyrolysis residue (solid-bitumen reflectance [BRo] of 0.52%). (B) Solid bitumen as a groundmass and embayed by euhedral mineral faces (arrows pointing to solid bitumen at margins of carbonate in inset) in the Bakken Shale starting material (BRo of 0.32%). From Hackley and Cardott (2016). (C) Solid bitumen as a groundmass in the Ohio Shale Huron Member (Mbr.) in 340°C hydrous-pyrolysis residue (BRo of 1.02%). (D) Solid bitumen associated with foraminifera in Monterey Formation 320°C hydrous-pyrolysis residue (BRo of 0.61%).
to identify solid bitumen in shale sample starting materials and hydrous-pyrolysis residues. (A) Solid bitumen filling chambers of Globigerina foraminifera in Boquillas Formation (Eagle Ford Formation equivalent) marl in 320°C hydrous-pyrolysis residue (solid-bitumen reflectance [BRo] of 0.52%). (B) Solid bitumen as a groundmass and embayed by euhedral mineral faces (arrows pointing to solid bitumen at margins of carbonate in inset) in the Bakken Shale starting material (BRo of 0.32%). From Hackley and Cardott (2016). (C) Solid bitumen as a groundmass in the Ohio Shale Huron Member (Mbr.) in 340°C hydrous-pyrolysis residue (BRo of 1.02%). (D) Solid bitumen associated with foraminifera in Monterey Formation 320°C hydrous-pyrolysis residue (BRo of 0.61%).
Whole-sample geochemical analyses were determined on the original samples and their pyrolysis residues after powdering via mortar and pestle. Analyses included total organic carbon (TOC) content by LECO and Rock-Eval II and Hawk pyrolysis (analyzed at Weatherford and USGS in Denver) per typical methods (Espitalie et al., 1985). Samples were not run in duplicate, so variability in measurement could not be calculated; however, estimates of variability were generated from comparison with replicate analyses of similar samples from other studies. Error in temperature of maximum S2 output (Tmax) values is ±2°C–3°C, whereas error in TOC, S1 (thermal distillate, in milligrams of HC per gram of rock), S2 (thermally cracked HCs, milligrams of HC per gram of rock), and S3 (CO and CO2 from thermal decomposition of organic matter, milligrams of CO and CO2 per gram of rock) is ±10%.
RESULTS
Bulk Rock Analyses
Results of TOC analysis of shale starting materials indicate TOC values from 4.1 to 22.4 wt. % (Table 2), with the highest value occurring in the Eocene Green River Mahogany-zone oil shale and the lowest in the Upper Cretaceous Boquillas Formation. Coals have higher TOC values, ranging from 38 to 62 wt. %. The S1 thermal-distillate values range from 0.65 to 11.97 mg HC/g rock for all samples with values slightly higher on average in shales compared with coals, consistent with the presence of petroleum products in the form of solid bitumen. The S2 pyrolysis output values range from 29.7 to 195.0 mg HC/g rock with values higher on average in coals. The Tmax values are universally low, ranging from 414°C to 439°C, indicating that samples are immature (Peters, 1986); the highest value of 439°C is from the Mahogany-zone sample with type I kerogen, for which Tmax values generally are considered to be unreliable as a maturity indicator (Tissot et al., 1987; Huizinga et al., 1988). Hydrogen index (HI) values are highest in shales, averaging 583 mg HC/g TOC and ranging from 392 to 871 mg HC/g TOC, with the highest value in the Mahogany-zone sample and the lowest in the Mississippian Barnett Shale. The HI versus Tmax discriminant plot (Figure 3) indicates that the shales contain type II kerogen (excepting the type I Mahogany zone), consistent with marine depositional environments as known from prior studies summarized in Hackley and Cardott (2016). Coals show universally lower HI values, ranging from 87 to 240 mg HC/g TOC and averaging only 178 mg HC/g TOC, consistent with lower H/C ratios in type III kerogen. Production index (S1/[S1 + S2]) values are low for all samples, ranging from 0.01 to 0.11, consistent with immature conditions as revealed by the Tmax values of generally less than 435°C (Peters, 1986).

 Figure 3. Hydrogen index versus temperature of maximum S2 output (Tmax) plot (Espitalie et al., 1985) showing kerogen types and thermal maturity of sample starting materials. HC = hydrocarbon; Ro = vitrinite reflectance; TOC = total organic carbon.
Figure 3. Hydrogen index versus temperature of maximum S2 output (Tmax) plot (Espitalie et al., 1985) showing kerogen types and thermal maturity of sample starting materials. HC = hydrocarbon; Ro = vitrinite reflectance; TOC = total organic carbon.
Reflectance Measurements
Although all of the samples  used
used in this study are thermally immature as shown in Figure 3, mean BRo measurements (0.23%–0.41% BRo) are consistently lower than Ro in the coals (0.42%–0.54% VRo; Tables 3, 4). As shown in Figure 4A, the BRo and Ro measurements of hydrous-pyrolysis products show systematically different signatures with increasing temperature. For example, Ro of coal recovered from 300°C experiments shows a significant increase from the original low values up to 0.80%–0.97% Ro, whereas BRo values from 300°C experiments are only 0.33%–0.58% (except results from the Green River Mahogany-zone sample; see description below). At 320°C, Ro values increase to 1.03%–1.15%, with BRo only increasing to 0.46%–0.68%. At higher hydrous-pyrolysis temperatures, the differences between Ro and BRo decrease. At 340°C, Ro values are 1.08%–1.36% and BRo values are 0.71%–1.08%, and at 360°C, Ro values are 1.39%–1.57% and BRo values are 1.17%–1.45%, a gap of only 0.2%–0.3% compared with a gap of approximately 0.5% at 320°C. The Ro of recovered Carbondale coal from the 340°C and 350°C experiments is notably below the overall vitrinite trend, and the BRo of recovered Mahogany-zone oil shale from the 300°C, 320°C, and 340°C experiments is notably below the overall solid-bitumen trend (Figure 4A). Note that the native solid bitumen present in the original Mahogany-zone oil-shale sample (BRo of 0.33%) is not identified with confidence in the residues of hydrous-pyrolysis experiments; instead, a newly generated solid bitumen converted from the original amorphous kerogen during the experiment is present. The two overall trends in Figure 4A are similar to those observed by Lewan (1985, 1993a), as shown in Figure 4B. In these earlier studies, reflectance values were measured by an experienced petrographer (John Grayson) who considered all of the macerals to be syndepositional vitrinite based on their uniform reflectance and sharp outlines. As a result, these macerals were considered to have suppressed Ro, as originally described by Price and Barker (1985). A later study on the Cambrian–Ordovician Alum Shale (Buchardt and Lewan, 1990) showed that the suppressed vitrinite was more appropriately referred to as vitrinite-like maceral.
in this study are thermally immature as shown in Figure 3, mean BRo measurements (0.23%–0.41% BRo) are consistently lower than Ro in the coals (0.42%–0.54% VRo; Tables 3, 4). As shown in Figure 4A, the BRo and Ro measurements of hydrous-pyrolysis products show systematically different signatures with increasing temperature. For example, Ro of coal recovered from 300°C experiments shows a significant increase from the original low values up to 0.80%–0.97% Ro, whereas BRo values from 300°C experiments are only 0.33%–0.58% (except results from the Green River Mahogany-zone sample; see description below). At 320°C, Ro values increase to 1.03%–1.15%, with BRo only increasing to 0.46%–0.68%. At higher hydrous-pyrolysis temperatures, the differences between Ro and BRo decrease. At 340°C, Ro values are 1.08%–1.36% and BRo values are 0.71%–1.08%, and at 360°C, Ro values are 1.39%–1.57% and BRo values are 1.17%–1.45%, a gap of only 0.2%–0.3% compared with a gap of approximately 0.5% at 320°C. The Ro of recovered Carbondale coal from the 340°C and 350°C experiments is notably below the overall vitrinite trend, and the BRo of recovered Mahogany-zone oil shale from the 300°C, 320°C, and 340°C experiments is notably below the overall solid-bitumen trend (Figure 4A). Note that the native solid bitumen present in the original Mahogany-zone oil-shale sample (BRo of 0.33%) is not identified with confidence in the residues of hydrous-pyrolysis experiments; instead, a newly generated solid bitumen converted from the original amorphous kerogen during the experiment is present. The two overall trends in Figure 4A are similar to those observed by Lewan (1985, 1993a), as shown in Figure 4B. In these earlier studies, reflectance values were measured by an experienced petrographer (John Grayson) who considered all of the macerals to be syndepositional vitrinite based on their uniform reflectance and sharp outlines. As a result, these macerals were considered to have suppressed Ro, as originally described by Price and Barker (1985). A later study on the Cambrian–Ordovician Alum Shale (Buchardt and Lewan, 1990) showed that the suppressed vitrinite was more appropriately referred to as vitrinite-like maceral.
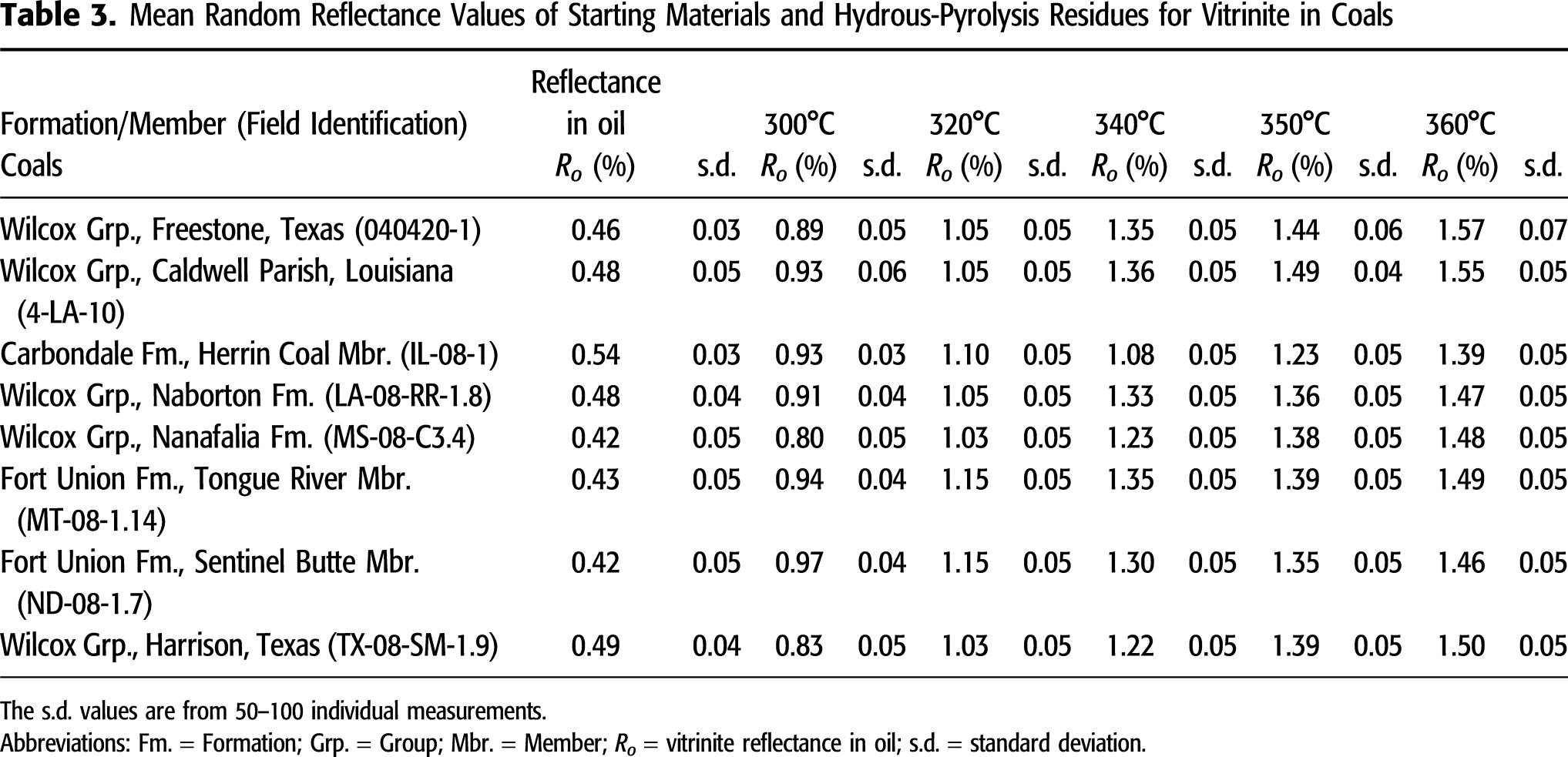
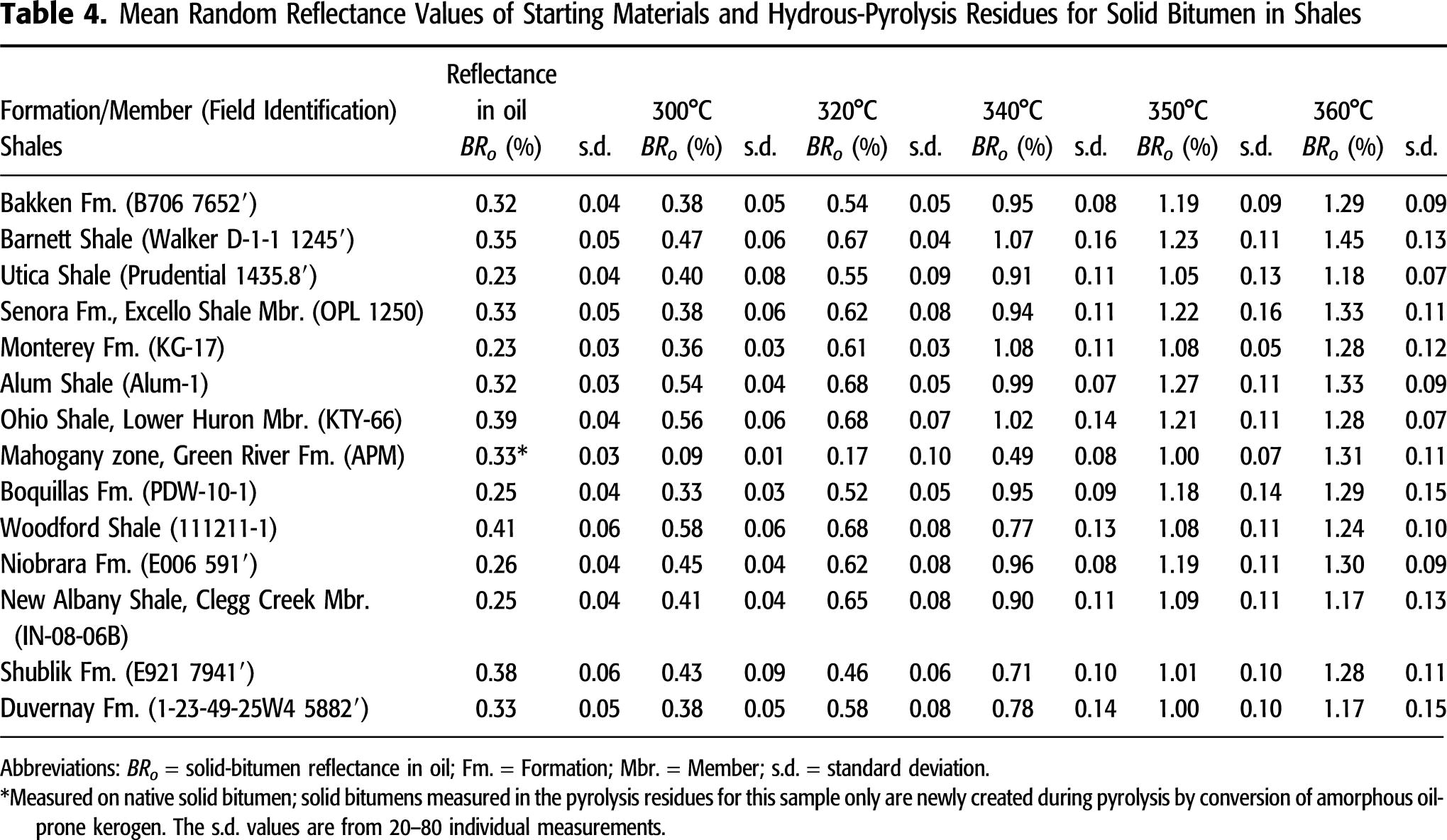
 Figure 4. Changes in reflectance of immature samples subjected to hydrous pyrolysis at various temperatures for 72 hr. (A) Results from this study in which reflectance was measured on petrographically identified vitrinite (Ro) in coals and solid bitumen (BRo) in shales. (B) Results from studies by Lewan (1985, 1993a) in which the two trends were identified as Ro and suppressed-Ro trends. Oven temperatures are assumed to be accurate to ±1°C.
Figure 4. Changes in reflectance of immature samples subjected to hydrous pyrolysis at various temperatures for 72 hr. (A) Results from this study in which reflectance was measured on petrographically identified vitrinite (Ro) in coals and solid bitumen (BRo) in shales. (B) Results from studies by Lewan (1985, 1993a) in which the two trends were identified as Ro and suppressed-Ro trends. Oven temperatures are assumed to be accurate to ±1°C.
Superimposing the data from this study (Figure 4A) with the previous work (Figure 4B) shows that the trends are similar but with some notable differences (Figure 5). The Ro values of the coals in this study are similar (Figure 5A) and group well within the vitrinite trend outlined by Lewan (1985, 1993a). Despite the wide variation in HI values for the initial immature coals (87–240 mg HC/g TOC), there appears to be little variability in the Ro of the coal recovered from the experiments. A notable exception from the coal Ro trend is the Carbondale Formation Herrin coal from the Illinois Basin. The Ro values for coal recovered from the 300°C and 320°C experiments are similar to the other coals and fall within the trend outlines for vitrinite. However, at 340°C, 350°C, and 360°C, the Ro of recovered Carbondale coal is significantly lower than the other coal samples and lower than the vitrinite trend outline. The Carbondale sample is the only Carboniferous coal in the study and the most mature of all the starting coal materials as determined by Ro. Its unique behavior in the higher-temperature experiments raises the question as to whether Carboniferous vitrinite responds differently to thermal stress than vitrinite from Cenozoic coals or its slightly higher initial thermal maturity causes it to be less reactive at higher experimental temperatures. In a study evaluating petroleum generation  potential
potential among more than 500 coals of various ages, Petersen (2006) noted that Cenozoic coals contained a greater proportion of long-chain aliphatic compounds than older coals. Collinson and Scott (1987) noted that vascular-plant precursors for coals of Carboniferous age are dominated by arborescent lycopsids, and coals of Cretaceous to Holocene are dominated by taxodiaceous conifers. However, Ernst and Mählmann (2004) found no difference in the maturation rate of angiosperm versus gymnosperm vitrinite.
among more than 500 coals of various ages, Petersen (2006) noted that Cenozoic coals contained a greater proportion of long-chain aliphatic compounds than older coals. Collinson and Scott (1987) noted that vascular-plant precursors for coals of Carboniferous age are dominated by arborescent lycopsids, and coals of Cretaceous to Holocene are dominated by taxodiaceous conifers. However, Ernst and Mählmann (2004) found no difference in the maturation rate of angiosperm versus gymnosperm vitrinite.
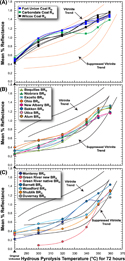 Figure 5. Changes in reflectance of immature samples subjected to hydrous pyrolysis at various temperatures for 72 hr reported in this study (colored symbols and curves) with vitrinite (Ro) and suppressed-Ro trends from Lewan (1985, 1993a) as shown in Figure 4B. (A) Results of Ro for coals from this study with spline curve fits. (B) Solid-bitumen reflectance (BRo) results for solid bitumen with sigmoid curves from this study. (C) Remaining BRo results from this study for solid bitumen, with some samples showing sigmoid curves as similar to (B) and other samples with more variable results. Oven temperatures are assumed to be accurate to ±1°C.
Figure 5. Changes in reflectance of immature samples subjected to hydrous pyrolysis at various temperatures for 72 hr reported in this study (colored symbols and curves) with vitrinite (Ro) and suppressed-Ro trends from Lewan (1985, 1993a) as shown in Figure 4B. (A) Results of Ro for coals from this study with spline curve fits. (B) Solid-bitumen reflectance (BRo) results for solid bitumen with sigmoid curves from this study. (C) Remaining BRo results from this study for solid bitumen, with some samples showing sigmoid curves as similar to (B) and other samples with more variable results. Oven temperatures are assumed to be accurate to ±1°C.
Vitrinite reflectance does not reach values greater than 1.57% Ro in this study, whereas Lewan (1993a) measured vitrinite values greater than 1.7% for one of two coal samples subjected to experimental temperatures of 360°C. In the Lewan (1993a) study, values of approximately 1.57% Ro were reached at 350°C for vitrinite in the Cretaceous Mowry Shale from Steinaker Draw, Utah. The experimental setup could be responsible for some difference in results. The current work  used
used minireactors with 25–35-ml internal volumes and small charges (2–4-g crushed rock, 13–16-ml water), whereas Lewan (1993a)
minireactors with 25–35-ml internal volumes and small charges (2–4-g crushed rock, 13–16-ml water), whereas Lewan (1993a)  used
used larger reactors with 1-L volume and up to 400-g crushed rock covered by water. Extended warm-up (1.1–1.5 hr) and cool-down (18–24 hr) times (Lewan and Ruble, 2002) of the larger reactors
larger reactors with 1-L volume and up to 400-g crushed rock covered by water. Extended warm-up (1.1–1.5 hr) and cool-down (18–24 hr) times (Lewan and Ruble, 2002) of the larger reactors  used
used by Lewan (1993a) could explain higher reflectance values of the previous work. Another consideration is that anisotropy of Ro may impact measurement precision at these higher maturities. Nevertheless, Ro is very similar in the two studies up to 340°C, and the values of BRo in this study and the suppressed-Ro values reported in Lewan (1993a) are similar at all experimental temperatures, with some outliers at 340°C.
by Lewan (1993a) could explain higher reflectance values of the previous work. Another consideration is that anisotropy of Ro may impact measurement precision at these higher maturities. Nevertheless, Ro is very similar in the two studies up to 340°C, and the values of BRo in this study and the suppressed-Ro values reported in Lewan (1993a) are similar at all experimental temperatures, with some outliers at 340°C.
Of the 14 shale samples, 11 have sigmoid BRo trends with temperature (Figure 5B, C). These trends are near or within the suppressed-Ro outline at 300°C, 320°C, 350°C, and 360°C but tend to have higher BRo values than the suppressed-Ro outline at 340°C. Shales with this sigmoid trend range in age from Cambrian–Ordovician through Cretaceous with no systematic relationship between their BRo and initial HI values (419–761 mg HC/g TOC). The remaining shale samples are shown with respect to temperature in Figure 5C. The BRo trends of the Shublik and Duvernay remain within the suppressed-Ro outline similar to the eight samples plotted in Figure 5B. The BRo trend of the Barnett Shale shows continuous increase with a concave trend throughout the entire temperature range. The Monterey Formation BRo trend shows a consistent concave increase and is higher than the suppressed-Ro outline to 340°C, but then it remains constant to 350°C with a subsequent increase to 360°C. The BRo trend for the Woodford Shale continuously increases with temperature. At 300°C and 320°C, it is above the suppressed-Ro outline, which was constructed with reflectance measurements made on the Woodford Shale in previous studies (Lewan, 1985, 1993a; Figure 4B). At 340°C, 350°C, and 360°C, its trend is within or bordering the suppressed-Ro outline. The BRo trend of the Mahogany-zone sample with temperature is the most anomalous, with BRo < 0.2% at 300°C and 320°C, which is less than solid bitumen in the initial sample (0.33% BRo). However, as noted above, the BRo measurements determined on rock recovered from hydrous pyrolysis of the Mahogany-zone sample were not on the native solid bitumen but instead from newly generated solid bitumen derived from conversion of the amorphous kerogen. At the higher experimental temperatures, the BRo trend of the Mahogany-zone sample increases into the suppressed-Ro outline.
The increase of BRo with experimental temperature is shown visually in Figure 6 in a series of images from rock samples recovered from hydrous pyrolysis of the Cambrian–Ordovician Alum Shale (Figure 6A–F) and the Devonian Huron Member of the Ohio Shale (Figure 6G–L). Pre-oil solid bitumen is evident from groundmass textures in the starting materials (Figure 6A, G), and its reflectance increase is visible in photomicrographs of the recovered rock and in the measured values (Tables 3, 4).
 Figure 6. Images of pre-oil solid bitumen (sb) in starting materials and sb in the recovered rock from hydrous pyrolysis (HP) of the Alum and Huron shales. All images taken with incident white light under oil immersion. (A) Alum Shale starting material. (B) Alum Shale recovered from 300°C HP experiment. (C) Alum Shale recovered from 320°C HP experiment. (D) Alum Shale recovered from 340°C HP experiment. (E) Alum Shale recovered from 350°C HP experiment. (F) Alum Shale recovered from 360°C HP experiment. (G) Huron Member of the Ohio Shale starting material. (H) Huron recovered from 300°C HP experiment. Tasmanites is a marine alga present in many of the late Paleozoic shales of North America (Hackley and Cardott, 2016). (I) Huron recovered from 320°C HP experiment. (J) Huron recovered from 340°C HP experiment. (K) Huron recovered from 350°C HP experiment. (L) Huron recovered from 360°C HP experiment. inert = inertinite.
Figure 6. Images of pre-oil solid bitumen (sb) in starting materials and sb in the recovered rock from hydrous pyrolysis (HP) of the Alum and Huron shales. All images taken with incident white light under oil immersion. (A) Alum Shale starting material. (B) Alum Shale recovered from 300°C HP experiment. (C) Alum Shale recovered from 320°C HP experiment. (D) Alum Shale recovered from 340°C HP experiment. (E) Alum Shale recovered from 350°C HP experiment. (F) Alum Shale recovered from 360°C HP experiment. (G) Huron Member of the Ohio Shale starting material. (H) Huron recovered from 300°C HP experiment. Tasmanites is a marine alga present in many of the late Paleozoic shales of North America (Hackley and Cardott, 2016). (I) Huron recovered from 320°C HP experiment. (J) Huron recovered from 340°C HP experiment. (K) Huron recovered from 350°C HP experiment. (L) Huron recovered from 360°C HP experiment. inert = inertinite.
DISCUSSION
Although the window for oil generation can vary significantly with Ro and kerogen type because of differing oil-generation kinetics (Higley et al., 2009), the two observed trends in this and prior studies (Lewan, 1985, 1993a) occur within the thermal-maturity range typically prescribed for oil generation (0.5%–1.3% Ro; Dow and O’Connor, 1982). Therefore, it is important to recognize whether reflectance measurements are on solid bitumen or vitrinite. As shown in this work, the reflectance of these two types of organic matter varied significantly when subjected to the same thermal stress as induced by hydrous pyrolysis (Figure 5). Visual differentiation of vitrinite from solid bitumen may be obvious in some cases, but the criteria are not always infallible. As noted in an interlaboratory reflectance-measurement study on shale (Hackley et al., 2015), reflectance values reported on organic matter identified as solid bitumen by some petrographers were identical with those measured on organic matter identified as vitrinite by other petrographers. As shown in this study, the hydrous-pyrolysis approach using temperatures between 300°C and 330°C for 72 hr could assist in distinguishing Ro from BRo in immature or low-maturity samples. However, with increasing thermal stress the Ro and BRo trends merge, and the specific hydrous-pyrolysis conditions for differentiating the two materials in higher-maturity rocks have not been studied. It should be noted that the immature shales of this study may have also contained vitrinite (Devonian and younger), but only the reflectance of solid bitumen (BRo) was recorded. The results from subjecting these shales to hydrous pyrolysis show that the reflectance measurements were not on vitrinite.
These data explain the results of empirical studies (Robert, 1988; Landis and Castaño, 1995; Ferreiro Mählmann and Frey, 2012; Ferreiro Mählmann and Le Bayon, 2016), which found that BRo is lower than co-occurring Ro at low thermal maturities (Ro < 1.0%) and more similar at higher maturities (Ro > 1.0%). Many workers have noted the possibility of Ro suppression in studies of upper Paleozoic–oil-window mature source rocks, including the Bakken Shale in the Williston Basin (Price and Barker, 1985; Jarvie et al., 2011) and Devonian shales in the Appalachian (Hackley et al., 2013; Ryder et al., 2013) and Illinois Basins (Barrows and Cluff, 1984; Nuccio and Hatch, 1996). Solid bitumen is the primary organic component in these and other currently developed shale petroleum reservoirs in North America (Hackley and Cardott, 2016), suggesting that some reports of Ro suppression simply are a consequence of mistaking solid bitumen for vitrinite. Therefore, it is suggested that Ro suppression is a misnomer in these upper Paleozoic marine-shale petroleum systems. Vitrinite-reflectance suppression also is widely reported from shale of other geologic ages (Mesozoic–Cenozoic) and from other depositional environments including fluvial-deltaic to lacustrine settings (Fatimah and Ward, 2009; Petersen et al., 2009; Wilkins et al., 2015; Hackley et al., 2016; Schito et al., 2016). The wide range in age (Cambrian–Ordovician to Miocene) of the shales  used
used in this study in which BRo consistently shows lower response to thermal stress than does Ro suggests that our work has application to a broader array of settings and ages, such as those mentioned above.
in this study in which BRo consistently shows lower response to thermal stress than does Ro suggests that our work has application to a broader array of settings and ages, such as those mentioned above.
Causes of Vitrinite-Reflectance Suppression
Many empirical geologic studies have reported Ro suppression in rock and sediment samples, where the suppression is a downward deviation from Ro values measured in associated samples that have a similar geologic history. This has often been explained as a consequence of association of the suppressed vitrinite with aliphatic, lipid-rich organic materials (liptinite), which may exsolve lower-reflecting oil or bitumen that impregnates vitrinite and lowers its reflectance (Kalkreuth, 1982; Kalkreuth and Macauley, 1987; Petersen and Vosgerau, 1999). However, some research (Barker et al., 2007) found no evidence for bitumen impregnation in a comparison of reflectance values for pre- and postextracted vitrinite. Hydrous pyrolysis of Mowry Shale and Cretaceous Frontier coal (also from Steinaker Draw, Utah) followed the same vitrinite trend in spite of significant oil generation from the former (Lewan, 1993a; Figure 4B). Efforts to deal with suppression issues include the use of empirical data to derive correction  functions
functions for application to suppressed-Ro values (Wilkins et al., 1992; Lo, 1993; Quick, 1994; Newman, 1997; Wilkins et al., 2002), although some have discounted this approach because of inconsistent results (Ryder et al., 2013). Others have proposed selection criteria for avoiding suppressed vitrinite (Buiskool Toxopeus, 1983); however, this approach presumes that normal vitrinite also is present in the samples that contain the suppressed vitrinite.
for application to suppressed-Ro values (Wilkins et al., 1992; Lo, 1993; Quick, 1994; Newman, 1997; Wilkins et al., 2002), although some have discounted this approach because of inconsistent results (Ryder et al., 2013). Others have proposed selection criteria for avoiding suppressed vitrinite (Buiskool Toxopeus, 1983); however, this approach presumes that normal vitrinite also is present in the samples that contain the suppressed vitrinite.
Work by Huang (1996) observed a control by HI on the evolution rate of Ro, where higher original hydrogen content caused a slower rate of Ro change in isothermally heated samples, with the effect becoming less pronounced at longer heating times (higher maturation). This result was consistent with observations of Ro suppression occurring in a hydrogen-rich vitrinite-like maceral noted in the empirical studies of earlier workers (Hutton and Cook, 1980; Price and Barker, 1985; Wenger and Baker, 1987). The current study does not support the claim, because coals of varying HI values (87–240 mg HC/g TOC) have similar vitrinite trends with hydrous-pyrolysis temperature (Figure 5A). Huang (1996) also found no control by fluid chemistry, fluid flow, gas or water pressure, or presence of water, oil, or other kerogen types on Ro evolution. We cannot address or refute these observations by Huang, because our experiments were invariant with the exception of temperature. However, we note that Huang’s conclusion concerning the presence of water is not consistent with later findings (Behar et al., 2003), which observed elevated increases in Ro in hydrous relative to anhydrous conditions. Behar et al. (2003) suggested that exogenous hydrogen derived from water may facilitate the vitrinite maturation reaction. Other experimental work showed a clear relation between H+ activity (i.e., pH) and the rate of increase of Ro (Seewald et al., 2000), also inconsistent to the findings of Huang (1996). Seewald et al. (2000) suggested that chemical maturation of vitrinite occurred via an acid-catalyzed mechanism. Similarly, the observation by Huang (1996) that water pressure has little effect on Ro evolution is in conflict with other experimental studies, which have observed retardation of reflectance evolution and oil generation under high-water-pressure conditions (Price and Wenger, 1992; Michels et al., 1995; Dalla Torre et al., 1997; Uguna et al., 2016b), and considerable controversy still exists on the factor of pressure (Le Bayon et al., 2011). A series of experimental studies by A. D. Carr and coworkers suggests that vitrinite maturation is retarded in overpressured systems where work is required to displace water and provide space for the volume expansion of kerogen and bitumen cracking (Carr et al., 2009; Uguna et al., 2012, 2013, 2016a, b). In their reasoning, the thermal energy  used
used to break organic bonds has to be divided between this thermal cracking and water displacement, reducing the overall amount of energy available for thermal cracking and thereby retarding organic maturation. We agree with the observation of Uguna et al. (2012) that differences in the experimental pyrolysis setup between studies may explain some of the conflicting results on the factor of pressure with respect to organic maturation and oil generation. In summary, a large body of work to date has produced conflicting and sometimes contradictory results on factors that may cause Ro suppression.
to break organic bonds has to be divided between this thermal cracking and water displacement, reducing the overall amount of energy available for thermal cracking and thereby retarding organic maturation. We agree with the observation of Uguna et al. (2012) that differences in the experimental pyrolysis setup between studies may explain some of the conflicting results on the factor of pressure with respect to organic maturation and oil generation. In summary, a large body of work to date has produced conflicting and sometimes contradictory results on factors that may cause Ro suppression.
The work reported herein suggests that the most likely cause for reports of Ro suppression is that the maceral being measured (solid bitumen) is compositionally different than vitrinite. Although its petrographic character (which for solid bitumen can include uniform reflectance and a sharp outline) may sometimes resemble vitrinite, solid bitumen is compositionally not vitrinite. As inferred from previous work, suppressed vitrinite (solid bitumen) may be enriched in hydrogen (Price and Barker, 1985; Hao and Chen, 1992; Ujiié et al., 2004), and it is intuitive that, as a solid HC product, solid bitumen would contain relatively more hydrogen than vitrinite. Although hydrogen enrichment is an attractive explanation for diminished reflectance values, it is difficult to substantiate. Bulk rock analyses are not appropriate, and the small amounts and sizes of dispersed macerals in shales make it difficult for direct compositional analyses of carbon and hydrogen to test the enriched hydrogen hypothesis for solid bitumen versus vitrinite. However, newly developed instruments may be capable of in situ chemical analyses at the small-length scales necessary (Cook et al., 2016; Yang et al., 2016), and this should be an important subject of future study.
The specific mechanism by which hydrogen enrichment inhibits reflectance increase in solid bitumen remains equivocal. One possibility is that hydrogen enrichment is manifested as aliphatic HC chains. The presence of these HC functional groups could inhibit the condensation of aromatic clusters, which is critical for the increase in reflectance with thermal maturation (Carr and Williamson, 1990). As these aliphatic functional groups are cracked from or cross-linked and aromatized into aromatic clusters, the condensation reactions responsible for reflectance increase would be allowed to proceed. This hypothesis could explain the observed merging of the two trends at hydrous-pyrolysis temperatures in excess of 330°C for 72 hr as seen in this work and in the prior studies (Lewan, 1985, 1993a).
As mentioned earlier, previous workers have noted the occurrence of vitrinite-like macerals, which are interpreted as syndepositional because of sharp outlines, uniform reflectance, and absence of void filling or groundmass textures (Houseknecht and Matthews, 1985; Buchardt and Lewan, 1990; Xiao et al., 2000; Schmidt et al., 2015). Buchardt and Lewan (1990) did not like the mobility connotation that solid bitumen conveys in describing the vitrinite-like maceral observed in rock thin sections and isolated-kerogen epoxy mounts of the Alum Shale. They contended that the dispersed character of this maceral within the immature rock groundmass and presence of framboidal pyrite in or around some of the individual vitrinite-like maceral fragments were indicative of a syndepositional origin and therefore not a diagenetic or catagenetic kerogen conversion product. They went on to suggest that in the same way gelification of cellulose and lignin from vascular plants could form vitrinite, gelification of algal polysaccharides may be the precursor of vitrinite-like macerals. We are not disputing this previous work or the possibility of identification of a vitrinite-like maceral by other workers, but instead we are showing different responses to thermal stress shown by vitrinite and solid bitumen as identified by unambiguous void filling or groundmass texture.
Implications to Petroleum Assessment
Assessments of petroleum generation rely on accurate burial-history reconstructions calibrated from Ro data. The work presented herein indicates that BRo will be lower than coexisting Ro, particularly at lower thermal maturities. Therefore, mistaken measurements of BRo in place of vitrinite will underestimate thermal maturity. The implication is that reconstructions of the thermal history of a source rock will be underestimated, and predictions of the extent of oil generation will be underestimated as well. In other words, recognition that Ro suppression is a misnomer potentially can extend the oil window and the possibility for oil accumulations into places where strata previously were expected to be immature for petroleum generation.
Applicable to both conventional and unconventional petroleum assessment, Ro measurements are  used
used to estimate the spatial extent (geographic location and depths) of oil and gas generation in the Earth’s crust (Suárez-Ruiz et al., 2012). Boundary locations for entrance to the oil window typically are placed at a minimum of 0.55%–0.60% Ro (Jarvie et al., 2005; Dembicki, 2009), although lower values are recognized for high-sulfur kerogen and higher values for type I kerogen (Orr, 1986; Baskin and Peters, 1992; Ruble et al., 2001). For workers evaluating thermally mature unconventional Paleozoic petroleum systems such as the Mississippian Barnett Shale in the Fort Worth Basin or the Devonian Appalachian Basin shales where solid bitumen is the dominant organic-matter component (Hackley and Cardott, 2016), assuming that the entrance to the oil window occurs at 0.60% Ro may decrease the areas in which self-sourced reservoirs are predicted. The USGS and other organizations (e.g., oil companies) sometimes use spatial extent (among other criteria) of 0.60% Ro conditions to estimate the volumes of undiscovered self-sourced petroleum resources reservoired in shale (Milici and Swezey, 2006; Coleman et al., 2011). If boundaries are underestimated because Ro measurements mistakenly include measurements of BRo, then estimated volumes of undiscovered HCs also will be underestimated. Therefore, identifying and distinguishing vitrinite from solid bitumen are critical.
to estimate the spatial extent (geographic location and depths) of oil and gas generation in the Earth’s crust (Suárez-Ruiz et al., 2012). Boundary locations for entrance to the oil window typically are placed at a minimum of 0.55%–0.60% Ro (Jarvie et al., 2005; Dembicki, 2009), although lower values are recognized for high-sulfur kerogen and higher values for type I kerogen (Orr, 1986; Baskin and Peters, 1992; Ruble et al., 2001). For workers evaluating thermally mature unconventional Paleozoic petroleum systems such as the Mississippian Barnett Shale in the Fort Worth Basin or the Devonian Appalachian Basin shales where solid bitumen is the dominant organic-matter component (Hackley and Cardott, 2016), assuming that the entrance to the oil window occurs at 0.60% Ro may decrease the areas in which self-sourced reservoirs are predicted. The USGS and other organizations (e.g., oil companies) sometimes use spatial extent (among other criteria) of 0.60% Ro conditions to estimate the volumes of undiscovered self-sourced petroleum resources reservoired in shale (Milici and Swezey, 2006; Coleman et al., 2011). If boundaries are underestimated because Ro measurements mistakenly include measurements of BRo, then estimated volumes of undiscovered HCs also will be underestimated. Therefore, identifying and distinguishing vitrinite from solid bitumen are critical.
Although this study found smaller differences in the reflectance of vitrinite and solid bitumen at higher experimental temperatures, some differences were noted. The observation that BRo < Ro at thermal maturities consistent with gas-condensate generation (1.1%–1.2% BRo) could suggest  potential
potential impact to models for thermogenic gas generation from oil cracking. A widely cited model of thermogenic processes in the Barnett Shale of the Fort Worth Basin originally suggested that oil cracking to secondary gas occurred at 1.1% Ro (Jarvie et al., 2005, 2007, their figure 11). Subsequent work that included new Ro measurements on additional samples (Lewan and Pawlewicz, 2017) indicates that the previously reported reflectance values (Pollastro et al., 2007) were significantly lower and most likely were the result of measurements on solid bitumen or misinterpretation of indigenous reflectance populations. As a result, oil cracking to secondary gas was reevaluated to occur at 1.5% Ro. Other workers have also noted onset of oil cracking at higher thermal maturities (Schenk et al., 1997; Waples, 2000), and this difference becomes important when using the Barnett Shale as an analog for other petroleum systems.
impact to models for thermogenic gas generation from oil cracking. A widely cited model of thermogenic processes in the Barnett Shale of the Fort Worth Basin originally suggested that oil cracking to secondary gas occurred at 1.1% Ro (Jarvie et al., 2005, 2007, their figure 11). Subsequent work that included new Ro measurements on additional samples (Lewan and Pawlewicz, 2017) indicates that the previously reported reflectance values (Pollastro et al., 2007) were significantly lower and most likely were the result of measurements on solid bitumen or misinterpretation of indigenous reflectance populations. As a result, oil cracking to secondary gas was reevaluated to occur at 1.5% Ro. Other workers have also noted onset of oil cracking at higher thermal maturities (Schenk et al., 1997; Waples, 2000), and this difference becomes important when using the Barnett Shale as an analog for other petroleum systems.
CONCLUSIONS
Results from hydrous pyrolysis of solid bitumen and vitrinite document differences in the rate of their reflectance increase with respect to thermal stress. Solid-bitumen reflectance shows a lower response to thermal stress than Ro, with differences most prominent at lower experimental temperatures and similarities most prominent at higher temperatures. These results explain empirical observations that BRo is lower than the reflectance of co-occurring vitrinite at low thermal maturities (Ro < 1.0%) and more similar at higher maturities (Ro > 1.0%). Optically distinguishing between solid bitumen and vitrinite may sometimes be difficult. In these cases, hydrous pyrolysis (300°C–330°C for 72 hr) of immature samples could help to determine whether observed vitrinite-like macerals are solid bitumen or vitrinite by their change in reflectance.
Because previous workers have shown that solid bitumen is a common organic-matter constituent in the thermally mature shale plays of North America (Hackley and Cardott, 2016), mistaken inclusion of lower-reflectance solid bitumen in the Ro histograms from these rocks may result in erroneous reports of Ro suppression. Such reports are common from upper Paleozoic marine shales showing early to mid-oil window maturity where vitrinite is rare or absent and solid bitumen is prevalent. Therefore, it is suggested that Ro suppression is a misnomer in the upper Paleozoic shale petroleum systems of North America and perhaps elsewhere in strata from other geologic time periods and depositional environments. This observation may be  used
used to expand the spatial boundaries in which petroleum generation could be expected from source rocks otherwise thought to be immature.
to expand the spatial boundaries in which petroleum generation could be expected from source rocks otherwise thought to be immature.
REFERENCES CITED
ASTM International, 2015a, ASTM D2797/D2797M-11a: Standard practice for preparing coal samples for microscopical analysis by reflected light: West Conshohocken, Pennsylvania, ASTM International, 5 p., doi:10.1520/D2797_D2797M-11A.
ASTM International, 2015b, ASTM D2798-11a: Standard test method for microscopical determination of the vitrinite reflectance of coal: West Conshohocken, Pennsylvania, ASTM International, 5 p., doi:10.1520/D2798-11A.
ASTM International, 2015c, ASTM D7708-14: Standard test method for microscopical determination of the reflectance of vitrinite dispersed in sedimentary rocks: West Conshohocken, Pennsylvania, ASTM International, 10 p., doi:10.1520/D7708-14.
Barker, C. E., M. D. Lewan, and M. J. Pawlewicz, 2007, The influence of extractable organic matter on vitrinite reflectance suppression: A survey of kerogen and coal types: International Journal of Coal Geology, v. 70, p. 67–78, doi:10.1016/j.coal.2006.03.005.
Barrows, M. H., and R. M. Cluff, 1984, New Albany Shale Group (Devonian-Mississippian) source rocks and hydrocarbon generation in the Illinois Basin, in G. Demaison and R. J. Murvis, eds., Petroleum geochemistry and basin evolution: AAPG Memoir 36, p. 111–138.
Baskin, D. K., and K. E. Peters, 1992, Early generation characteristics of a sulfur-rich Monterey kerogen: AAPG Bulletin, v. 76, no. 1, p. 1–13.
Behar, F., M. D. Lewan, F. Lorant, and M. Vandenbroucke, 2003, Comparison of artificial maturation of lignite in hydrous and nonhydrous conditions: Organic Geochemistry, v. 34, p. 575–600, doi:10.1016/S0146-6380(02)00241-3.
Buchardt, B., and M. D. Lewan, 1990, Reflectance of vitrinite-like macerals as a thermal maturity index for Cambrian-Ordovician Alum Shale, southern Scandinavia: AAPG Bulletin, v. 74, no. 4, p. 394–406.
Buiskool Toxopeus, J. M. A., 1983, Selection criteria for the use of vitrinite reflectance as a maturity tool, in Brooks, J., ed., Petroleum geochemistry and exploration of Europe: Geological Society, London, Special Publications 1983, v. 12, p. 295–307, doi:10.1144/GSL.SP.1983.012.01.30.
Cardott, B. J., C. R. Landis, and M. E. Curtis, 2015, Post-oil solid bitumen network in the Woodford Shale, USA—A  potential
potential primary migration pathway: International Journal of Coal Geology, v. 139, p. 106–113, doi:10.1016/j.coal.2014.08.012.
primary migration pathway: International Journal of Coal Geology, v. 139, p. 106–113, doi:10.1016/j.coal.2014.08.012.
Carr, A. D., 2000, Suppression and retardation of vitrinite reflectance, part 1: Formation and significance for hydrocarbon generation: Journal of Petroleum Geology, v. 23, p. 313–343, doi:10.1111/j.1747-5457.2000.tb01022.x.
Carr, A. D., C. E. Snape, W. Meredith, C. Uguna, I. C. Scotchman, and R. C. Davis, 2009, The effect of water pressure on hydrocarbon generation reactions: Some inferences from laboratory experiments: Petroleum Geoscience, v. 15, p. 17–26, doi:10.1144/1354-079309-797.
Carr, A. D., and J. E. Williamson, 1990, The relationship between aromaticity, vitrinite reflectance and maceral composition of coals: Implications for the use of vitrinite reflectance as a maturation parameter: Organic Geochemistry, v. 16, p. 313–323, doi:10.1016/0146-6380(90)90051-Z.
Chaffee, A. L., R. B. Johns, M. J. Baerken, J. W. De Leeuw, P. A. Schenk, and J. J. Boon, 1984, Chemical effects in gelification processes and lithotype formation in Victorian brown coal: Organic Geochemistry, v. 6, p. 409–416, doi:10.1016/0146-6380(84)90063-9.
Coleman, J. L., R. C., Milici, T. A. Cook, R. R. Charpentier, M. Kirshbaum, T. R. Klett, R. M. Pollastro, and C. J. Schenk, 2011, Assessment of undiscovered oil and gas resources of the Devonian Marcellus Shale of the Appalachian Basin Province, 2011: US Geological Survey Fact Sheet 2011-3092, accessed June 21, 2017, https://pubs.usgs.gov/fs/2011/3092/pdf/fs2011-3092.pdf.
Collinson, M. E., and A. C. Scott, 1987, Implications of vegetational change through the geological record on models for coal-forming environments, in Scott, A. C., ed., Coal and coal-bearing strata: Recent advances: Geological Society, London, Special Publication 1987, v. 32, p. 67–85, doi:10.1144/GSL.SP.1987.032.01.06.
Cook, D., P. C. Hackley, K. Kjoller, D. Dawson, R. Shetty, and E. Dillon, 2016, Investigating oil-prone kerogen conversion to hydrocarbons using AFM-based infrared spectroscopy (abs.): American Geophysical Union Fall Meeting, San Francisco, December 12–16, 2016, accessed June 17, 2016, https://agu.confex.com/agu/fm16/meetingapp.cgi/Paper/187753.
Curiale, J. A., 1986, Origin of solid bitumens, with emphasis on biological marker results: Organic Geochemistry, v. 10, p. 559–580, doi:10.1016/0146-6380(86)90054-9.
Dalla Torre, M., R. F. Mählmann, and W. G. Ernst, 1997, Experimental study on the pressure dependence of vitrinite maturation: Geochimica et Cosmochimica Acta, v. 61, p. 2921–2928, doi:10.1016/S0016-7037(97)00104-X.
Dembicki, H., 2009, Three common source rock evaluation errors made by geologists during prospect or play appraisals: AAPG Bulletin, v. 93, no. 3, p. 341–356, doi:10.1306/10230808076.
Dow, W. G., 1977, Kerogen studies and geological interpretations: Journal of Geochemical Exploration, v. 7, p. 79–99, doi:10.1016/0375-6742(77)90078-4.
Dow, W. G., and D. I. O’Connor, 1982, Kerogen maturity and type by reflected light microscopy applied to petroleum exploration, in F. L. Staplin, W. G. Dow, C. W. D. Milner, D. I. O’Connor, S. A. J. Pocock, P. van Gijzel, D. H. Welte, and M. A. Yükler, eds., How to assess maturation and paleotemperatures: Tulsa, Oklahoma, SEPM Short Course Number 7, p. 133–157, doi:10.2110/scn.82.07.0133.
Ernst, W. G., and R. F. Mählmann, 2004, Vitrinite alteration as a function of temperature, time, starting material, aqueous fluid pressure and oxygen fugacity: Laboratory corroboration of prior work, in R. J. Hill, J. Leventhal, Z. Aizenshtat, M. J. Baedecker, G. Claypool, R. Eganhouse, M. Goldhaber, and K. E. Peters, eds., Geochemical investigations in Earth and space science: A tribute to Isaac R. Kaplan: Amsterdam, The Geochemical Society Publication 9, p. 341–357, doi:10.1016/S1873-9881(04)80024-5.
Espitalie, J., G. Deroo, and F. Marquis, 1985, Rock-Eval pyrolysis and its applications: Revue de l’Institut Français du Pétrole, v. 40, p. 563–579, doi:10.2516/ogst:1985035.
Fatimah, and C. R. Ward, 2009, Mineralogy and organic petrology of oil shales in the Sangkarewang Formation, Ombilin Basin, West Sumatra, Indonesia: International Journal of Coal Geology, v. 77, p. 424–435, doi:10.1016/j.coal.2008.04.005.
Ferreiro Mählmann, R., and M. Frey, 2012, Standardisation, calibration and correlation of the Kübler-index and the vitrinite/bituminite reflectance: An inter-laboratory and field related study: Swiss Journal of Geosciences, v. 105, p. 153–170, doi:10.1007/s00015-012-0110-8.
Ferreiro Mählmann, R., and R. Le Bayon, 2016, Vitrinite and vitrinite like solid bitumen reflectance in thermal maturity studies: Correlations from diagenesis to incipient metamorphism in different geodynamic settings: International Journal of Coal Geology, v. 157, p. 52–73, doi:10.1016/j.coal.2015.12.008.
Hackley, P. C., 2012, Geological and geochemical characterization of the Lower Cretaceous Pearsall Formation, Maverick Basin, south Texas: A future shale gas resource?: AAPG Bulletin, v. 96, no. 8, p. 1449–1482, doi:10.1306/11221111071.
Hackley, P. C., C. V. Araujo, A. G. Borrego, A. Bouzinos, B. J. Cardott, A. C. Cook, and C. Eble, et al, 2015, Standardization of reflectance measurements in dispersed organic matter: Results of an exercise to improve interlaboratory agreement: Marine and Petroleum Geology, v. 59, p. 22–34, doi:10.1016/j.marpetgeo.2014.07.015.
Hackley, P. C., and B. J. Cardott, 2016, Application of organic petrography in North American shale petroleum systems: A review: International Journal of Coal Geology, v. 163, p. 8–51, doi:10.1016/j.coal.2016.06.010.
Hackley, P. C., N. Fishman, T. Wu, and G. Baugher, 2016, Organic petrology and geochemistry of mudrocks from the lacustrine Lucaogou Formation, Santanghu Basin, northwest China: Application to lake basin evolution: International Journal of Coal Geology, v. 168, p. 20–34, doi:10.1016/j.coal.2016.05.011.
Hackley, P. C., R. T. Ryder, M. H. Trippi, and H. Alimi, 2013, Thermal maturity of northern Appalachian Basin Devonian shales: Insights from sterane and terpane biomarkers: Fuel, v. 106, p. 455–462, doi:10.1016/j.fuel.2012.12.032.
Hao, F., and J. Chen, 1992, The cause and mechanism of vitrinite reflectance anomalies: Journal of Petroleum Geology, v. 15, p. 419–434, doi:10.1111/j.1747-5457.1992.tb01043.x.
Hao, F., Y. C. Sun, S. T. Li, and Q. M. Zhang, 1995, Overpressure retardation of organic-matter maturation and petroleum generation: A case study from the Yinggehai and Qiongdongnan Basins, South China Sea: AAPG Bulletin, v. 79, no. 4, p. 551–562.
Hao, F., H. Zou, Z. Gong, S. Yang, and Z. Zeng, 2007, Hierarchies of overpressure retardation of organic matter maturation: Case studies from petroleum basins in China: AAPG Bulletin, v. 91, no. 10, p. 1467–1498, doi:10.1306/05210705161.
Higley, D. K., M. D. Lewan, L. N. R. Roberts, and M. Henry, 2009, Timing and petroleum sources for the Lower Cretaceous Mannville Group oil sands of northern Alberta based on 4-D modeling: AAPG Bulletin, v. 93, no. 2, p. 203–230, doi:10.1306/09150808060.
Houseknecht, D. W., and S. M. Matthews, 1985, Thermal maturity of carboniferous strata, Ouachita Mountains: AAPG Bulletin, v. 69, no. 3, p. 335–345.
Huang, W. L., 1996, Experimental study of vitrinite maturation: Effects of temperature, time, pressure, water, and hydrogen index: Organic Geochemistry, v. 24, p. 233–241, doi:10.1016/0146-6380(96)00032-0.
Huizinga, B. J., Z. A. Aizenshtat, and K. E. Peters, 1988, Programmed pyrolysis-gas chromatography of artificially matured Green River kerogen: Energy & Fuels, v. 2, p. 74–81, doi:10.1021/ef00007a011.
Hunt, J. M., 1996, Petroleum geochemistry and geology, 2nd ed.: New York, W.H. Freeman and Company, 743 p.
Hutton, A. C., and A. C. Cook, 1980, Influence of alginite on the reflectance of vitrinite: Fuel, v. 59, p. 711–714, doi:10.1016/0016-2361(80)90025-3.
Jarvie, D. M., R. J. Coskey, M. S. Johnson, and J. E. Leonard, 2011, The geology and geochemistry of the Parshall area, Mountrail County, North Dakota, in J. W. Robinson, J. A. LeFever, and S. B. Gaswirth, eds., The Bakken–Three Forks petroleum system in the Williston Basin: Denver, Colorado, Rocky Mountain Association of Geologists, p. 229–268.
Jarvie, D. M., R. J. Hill, and R. M. Pollastro, 2005, Assessment of the gas  potential
potential and yields from shales: The Barnett Shale model, in B. J. Cardott, ed., Unconventional energy resources in the southern Midcontinent, 2004 symposium: Norman, Oklahoma, Oklahoma Geological Survey Circular 110, p. 37–50.
and yields from shales: The Barnett Shale model, in B. J. Cardott, ed., Unconventional energy resources in the southern Midcontinent, 2004 symposium: Norman, Oklahoma, Oklahoma Geological Survey Circular 110, p. 37–50.
Jarvie, D. M., R. J. Hill, T. E. Ruble, and R. M. Pollastro, 2007, Unconventional shale-gas systems: The Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment: AAPG Bulletin, v. 91, no. 4, p. 475–499, doi:10.1306/12190606068.
Kalkreuth, W., 1982, Rank and petrographic composition of selected Jurassic-Lower Cretaceous coals of British Columbia, Canada: Bulletin of Canadian Petroleum Geology, v. 30, p. 112–139.
Kalkreuth, W., and G. Macauley, 1987, Organic petrology and geochemical (Rock-Eval) studies on oil shales and coals from the Pictou and Antigonish areas, Nova Scotia, Canada: Bulletin of Canadian Petroleum Geology, v. 35, p. 263–295.
Landis, C. R., and J. R. Castaño, 1995, Maturation and bulk chemical properties of a suite of solid hydrocarbons: Organic Geochemistry, v. 22, p. 137–149, doi:10.1016/0146-6380(95)90013-6.
Le Bayon, R., G. P. Brey, W. G. Ernst, and R. F. Mahlmann, 2011, Experimental kinetic study of organic matter maturation: Time and pressure effects on vitrinite reflectance at 400°C: Organic Geochemistry, v. 42, p. 340–355, doi:10.1016/j.orggeochem.2011.01.011.
Levine, J. R., and A. Davis, 1984, Optical anisotropy of coals as an indicator of tectonic deformation, broad top coal field, Pennsylvania: Geological Society of America Bulletin, v. 95, p. 100–108, doi:10.1130/0016-7606(1984)95<100:OAOCAA>2.0.CO;2.
Lewan, M. D., 1985, Evaluation of petroleum generation by hydrous pyrolysis experimentation: Philosophical Transactions of the Royal Society of London, v. 315, p. 123–134, doi:10.1098/rsta.1985.0033.
Lewan, M. D., 1987, Petrographic study of primary petroleum migration in the Woodford shale and related rock units, in Doligez, B., ed., Migration of hydrocarbons in sedimentary basins: Paris, Editions Technip, p. 113–130.
Lewan, M. D., 1993a, Identifying and understanding suppressed vitrinite reflectance through hydrous pyrolysis experiments (abs.): Society for Organic Petrology 10th Annual Meeting, Norman, Oklahoma, October 9–13, 1993, p. 1–3.
Lewan, M. D., 1993b, Laboratory simulation of petroleum formation: Hydrous pyrolysis, in M. H. Engel and S. S. Macko, eds., Organic geochemistry: Principles and applications: New York, Plenum Press, p. 419–442, doi:10.1007/978-1-4615-2890-6_18.
Lewan, M. D., 1997, Experiments on the role of water in petroleum formation: Geochimica et Cosmochimica Acta, v. 61, p. 3691–3723, doi:10.1016/S0016-7037(97)00176-2.
Lewan, M. D., and M. J. Pawlewicz, 2017, Reevaluation of thermal maturity and stages of petroleum formation of the Mississippian Barnett Shale, Fort Worth Basin, Texas: AAPG Bulletin, v. 101, no. 12, p. 1945–1970, doi:10.1306/01251716053.
Lewan, M. D., and T. E. Ruble, 2002, Comparison of petroleum generation kinetics by isothermal hydrous and nonisothermal open-system pyrolysis: Organic Geochemistry, v. 33, p. 1457–1475, doi:10.1016/S0146-6380(02)00182-1.
Li, H., T. Wu, Z. Ma, and W. Zhang, 2004, Pressure retardation of organic maturation in clastic reservoirs: A case study from the Banqiao Sag, Eastern China: Marine and Petroleum Geology, v. 21, p. 1083–1093, doi:10.1016/j.marpetgeo.2004.07.005.
Lo, H. B., 1993, Correction criteria for the suppression of vitrinite reflectance in hydrogen-rich kerogen: Preliminary guidelines: Organic Geochemistry, v. 20, p. 653–657, doi:10.1016/0146-6380(93)90051-C.
McCartney, J. T., and M. Teichmüller, 1972, Classification of coals according to degree of coalification by reflectance of the vitrinite component: Fuel, v. 51, p. 64–68, doi:10.1016/0016-2361(72)90041-5.
McTavish, R. A., 1978, Pressure retardation of vitrinite diagenesis, offshore northwest Europe: Nature, v. 271, p. 648–650, doi:10.1038/271648a0.
McTavish, R. A., 1998, The role of overpressure in the retardation of organic matter maturation: Journal of Petroleum Geology, v. 21, p. 153–186, doi:10.1111/j.1747-5457.1998.tb00652.x.
Michels, R., P. Landais, B. E. Torkelson, and R. P. Philp, 1995, Effects of effluents and water pressure on oil generation during confined pyrolysis and high-pressure hydrous pyrolysis: Geochimica et Cosmochimica Acta, v. 59, p. 1589–1604, doi:10.1016/0016-7037(95)00065-8.
Milici, R. C., and C. S. Swezey, 2006, Assessment of Appalachian Basin oil and gas resources: Devonian Shale–Middle and Upper Paleozoic total petroleum system: Reston, Virginia, US Geological Survey Open-File Report 2006-1237, accessed June 12, 2017, https://pubs.usgs.gov/of/2006/1237/of2006-1237.pdf.
Mukhopadhyay, P. K., and W. G. Dow, eds, 1994, Vitrinite reflectance as a maturity parameter: Applications and limitations: Washington, DC, American Chemical Society Symposium Series, v. 570, 294 p., doi:10.1021/bk-1994-0570.
Newman, J., 1997, New approaches to detection and correction of suppressed vitrinite reflectance: Australian Petroleum Production and Exploration Association Journal, v. 37, p. 524–535.
Nuccio, V. F., and J. R. Hatch, 1996, Vitrinite reflectance suppression in the New Albany Shale, Illinois Basin: Vitrinite reflectance and Rock-Eval data: Reston, Virgina, US Geological Survey Open-File Report 96–665, accessed June 12, 2017, https://pubs.usgs.gov/of/1996/0665/report.pdf.
Orr, W. L., 1986, Kerogen/asphaltene/sulfur relationships in sulfur-rich Monterey oils: Organic Geochemistry, v. 10, p. 499–516, doi:10.1016/0146-6380(86)90049-5.
Peters, K. E., 1986, Guidelines for evaluating petroleum source rock using programmed pyrolysis: AAPG Bulletin, v. 70, no. 3, p. 318–329.
Petersen, H. I., 2006, The petroleum generation  potential
potential and effective oil window of humic coals related to coal composition and age: International Journal of Coal Geology, v. 67, p. 221–248, doi:10.1016/j.coal.2006.01.005.
and effective oil window of humic coals related to coal composition and age: International Journal of Coal Geology, v. 67, p. 221–248, doi:10.1016/j.coal.2006.01.005.
Petersen, H. I., N. H. Schovsbo, and A. T. Nielsen, 2013, Reflectance measurements of zooclasts and solid bitumen in Lower Paleozoic shales, southern Scandinavia: Correlation to vitrinite reflectance: International Journal of Coal Geology, v. 114, p. 1–18, doi:10.1016/j.coal.2013.03.013.
Petersen, H. I., N. Sherwood, A. Mathiesen, M. B. W. Fyhn, N. T. Dau, N. Russell, J. A. Bojesen-Koefoed, and L. H. Nielsen, 2009, Application of integrated vitrinite reflectance and FAMM analyses for thermal maturity assessment of the northeastern Malay Basin, offshore Vietnam: Implications for petroleum prospectivity evaluation: Marine and Petroleum Geology, v. 26, p. 319–332, doi:10.1016/j.marpetgeo.2008.04.004.
Petersen, H. I., and H. Vosgerau, 1999, Composition and organic maturity of Middle Jurassic coals, northeast Greenland: Evidence for liptinite-induced suppression of huminite reflectance: International Journal of Coal Geology, v. 41, p. 257–274, doi:10.1016/S0166-5162(99)00022-1.
Pollastro, R. M., D. M. Jarvie, R. J. Hill, and C. W. Adams, 2007, Geologic framework of the Mississippian Barnett Shale, Barnett-Paleozoic total petroleum system, Bend Arch-Fort Worth Basin, Texas: AAPG Bulletin, v. 91, no. 4, p. 405–436, doi:10.1306/10300606008.
Price, L. C., and C. E. Barker, 1985, Suppression of vitrinite reflectance in amorphous rich kerogen: A major unrecognized problem: Journal of Petroleum Geology, v. 8, p. 59–84, doi:10.1111/j.1747-5457.1985.tb00191.x.
Price, L. C., and L. M. Wenger, 1992, The influence of pressure on petroleum generation and maturation as suggested by aqueous pyrolysis: Organic Geochemistry, v. 19, p. 141–159, doi:10.1016/0146-6380(92)90033-T.
Quick, J. C., 1994, Iso-rank variation of vitrinite reflectance and fluorescence intensity, in P. K. Mukhopadhyay and W. G. Dow, eds., Vitrinite reflectance as a maturity parameter: Applications and limitations: Washington, DC, American Chemical Society Symposium Series 570, p. 64–75, doi:10.1021/bk-1994-0570.ch005.
Riediger, C. L., 1993, Solid bitumen reflectance and Rock-Eval Tmax as maturation indices: An example from the “Nordegg Member”, Western Canada Sedimentary Basin: International Journal of Coal Geology, v. 22, p. 295–315, doi:10.1016/0166-5162(93)90031-5.
Robert, P., 1988, Organic metamorphism and geothermal history: Microscopic study of organic matter and thermal evolution of sedimentary basins: Dordrecht, The Netherlands, Elf-Aquitaine and Reidel Publishing Company, 311 p.
Ruble, T. E., M. D. Lewan, and R. P. Philp, 2001, New Insights on the Green River petroleum system in the Uinta basin from hydrous pyrolysis experiments: AAPG Bulletin, v. 85, no. 8, p. 1333–1371.
Russell, N. J., 1984, Gelification of Victorian Tertiary soft brown coal wood. I. Relationship between chemical composition and microscopic appearance and variation in the degree of gelification: International Journal of Coal Geology, v. 4, p. 99–118, doi:10.1016/0166-5162(84)90010-7.
Ryder, R. T., P. C. Hackley, A. Hossein, and M. H. Trippi, 2013, Evaluation of thermal maturity in the low maturity Devonian shales of the northern Appalachian Basin:AAPG Search and Discovery article 10477, accessed June 12, 2017, http://www.searchanddiscovery.com/documents/2013/10477ryder/ndx_ryder.pdf.
Schenk, H. J., R. Di Primio, and B. Horsfield, 1997, The conversion of oil into gas in petroleum reservoirs. Part 1: Comparative kinetic investigation of gas generation from crude oils of lacustrine, marine and fluviodeltaic origin by programmed-temperature closed-system pyrolysis: Organic Geochemistry, v. 26, p. 467–481, doi:10.1016/S0146-6380(97)00024-7.
Schito, A., S. Corrado, L. Aldega, and D. Grigo, 2016, Overcoming pitfalls of vitrinite reflectance measurements in the assessment of thermal maturity: The case history of the lower Congo basin: Marine and Petroleum Geology, v. 74, p. 59–70, doi:10.1016/j.marpetgeo.2016.04.002.
Schmidt, J. S., C. V. Araujo, I. V. A. F. Souza, and R. B. A. Chagas, 2015, Hydrous pyrolysis maturation of vitrinite-like and humic vitrinite macerals: Implications for thermal maturity analysis: International Journal of Coal Geology, v. 144–145, p. 5–14, doi:10.1016/j.coal.2015.03.016.
Schoenherr, J., R. Littke, J. L. Urai, P. A. Kukla, and Z. Rawahi, 2007, Polyphase thermal evolution in the Infra-Cambrian Ara Group (South Oman Salt Basin) as deduced by maturity of solid reservoir bitumen: Organic Geochemistry, v. 38, p. 1293–1318, doi:10.1016/j.orggeochem.2007.03.010.
Seewald, J. S., L. B. Eglinton, and Y. L. Ong, 2000, An experimental study of organic-inorganic interactions during vitrinite maturation: Geochimica et Cosmochimica Acta, v. 64, p. 1577–1591, doi:10.1016/S0016-7037(00)00339-2.
Stout, S. A., and W. Spackman, 1987, A microscopic investigation of woody tissues in peats: Some processes active in the peatification of ligno-cellulosic cell walls: International Journal of Coal Geology, v. 8, p. 55–68, doi:10.1016/0166-5162(87)90022-X.
Suárez-Ruiz, I., D. Flores, J. G. Mendonça Filho, and P. C. Hackley, 2012, Review and update of the applications of organic petrology: Part 1, geological applications: International Journal of Coal Geology, v. 99, p. 54–112, doi:10.1016/j.coal.2012.02.004.
Taylor, G. H., M. Teichmüller, A. Davis, C. F. K. Diessel, R. Littke, and P. Robert, 1998, Organic petrology: Berlin, Gebruder Borntraeger, 704 p.
Tissot, B. P., R. Pelet, and P. Ungerer, 1987, Thermal history of sedimentary basins, maturation indices, and kinetics of oil and gas generation: AAPG Bulletin, v. 71, no. 12, p. 1445–1466.
Tissot, B. P., and D. H. Welte, 1984, Petroleum formation and occurrence, 2nd ed.: Berlin, Springer-Verlag, 699 p., doi:10.1007/978-3-642-87813-8.
Uguna, C. N., M. H. Azri, C. E. Snape, W. Meredith, and A. D. Carr, 2013, A hydrous pyrolysis study to ascertain how gas yields and the extent of maturation for a partially matured source rock and bitumen in isolation compared to their whole source rock: Journal of Analytical and Applied Pyrolysis, v. 103, p. 268–277, doi:10.1016/j.jaap.2012.11.007.
Uguna, C. N., A. D. Carr, C. E. Snape, and W. Meredith, 2016a, Retardation of oil cracking to gas and pressure induced combination reactions to account for viscous oil in deep petroleum basins: Evidence from oil and n-hexadecane pyrolysis at water pressures up to 900 bar: Organic Geochemistry, v. 97, p. 61–73, doi:10.1016/j.orggeochem.2016.04.007.
Uguna, C. N., A. D. Carr, C. E. Snape, W. Meredith, and M. Castro-Díaz, 2012, A laboratory pyrolysis study to investigate the effect of water pressure on hydrocarbon generation and maturation of coals in geological basins: Organic Geochemistry, v. 52, p. 103–113, doi:10.1016/j.orggeochem.2012.09.003.
Uguna, C. N., A. D. Carr, C. E. Snape, W. Meredith, I. C. Scotchman, A. Murray, and C. H. Vane, 2016b, Impact of high water pressure on oil generation and maturation in Kimmeridge Clay and Monterey source rocks: Implications for petroleum retention and gas generation in shale gas systems: Marine and Petroleum Geology, v. 73, p. 72–85, doi:10.1016/j.marpetgeo.2016.02.028.
Ujiié, Y., N. Sherwood, M. Faiz, and R. W. T. Wilkins, 2004, Thermal maturity and suppressed vitrinite reflectance for Neogene petroleum source rocks of Japan: AAPG Bulletin, v. 88, no. 10, p. 1335–1356, doi:10.1306/05030403035.
Valentine, B. J., P. C. Hackley, C. B. Enomoto, A. M. Bove, F. T. Dulong, C. D. Lohr, and K. R. Scott, 2014, Organic petrology of the Aptian-age section in the downdip Mississippi Interior Salt Basin, Mississippi, USA: Observations and preliminary implications for thermal maturation history: International Journal of Coal Geology, v. 131, p. 378–391, doi:10.1016/j.coal.2014.07.001.
Waples, D. W., 2000, The kinetics of in-reservoir oil destruction and gas formation: Constraints from experimental and empirical data, and from thermodynamics: Organic Geochemistry, v. 31, p. 553–575, doi:10.1016/S0146-6380(00)00023-1.
Wei, L., Y. Wang, and M. Mastalerz, 2016, Comparative optical properties of macerals and statistical evaluation of mis-identification of vitrinite and solid bitumen from early mature Middle Devonian–Lower Mississippian New Albany Shale: Implications for thermal maturity assessment: International Journal of Coal Geology, v. 168, p. 222–236, doi:10.1016/j.coal.2016.11.003.
Wenger, L. M., and D. R. Baker, 1987, Variations in vitrinite reflectance with organic facies: Examples from Pennsylvanian cyclothems of the midcontinent, USA: Organic Geochemistry, v. 11, p. 411–416, doi:10.1016/0146-6380(87)90075-1.
Wilkins, R. W. T., C. F. K. Diessel, and C. Buckingham, 2002, Comparison of two petrographic methods for determining the degree of anomalous vitrinite reflectance: International Journal of Coal Geology, v. 52, p. 45–62, doi:10.1016/S0166-5162(02)00110-6.
Wilkins, R. W. T., M. Wang, H. Gan, and Z. Li, 2015, A RaMM study of thermal maturity of dispersed organic matter in marine source rocks: International Journal of Coal Geology, v. 150–151, p. 252–264, doi:10.1016/j.coal.2015.09.007.
Wilkins, R. W. T., J. R. Wilmhurst, N. J. Russell, G. Hladky, M. V. Ellacott, and C. Buckingham, 1992, Fluorescence alteration and the suppression of vitrinite reflectance: Organic Geochemistry, v. 18, p. 629–640, doi:10.1016/0146-6380(92)90088-F.
Xiao, X., R. W. T. Wilkins, D. Liu, Z. Liu, and J. Fu, 2000, Investigation of thermal maturity of lower Paleozoic hydrocarbon source rocks by means of vitrinite-like maceral reflectance: A Tarim Basin case study: Organic Geochemistry, v. 31, p. 1041–1052, doi:10.1016/S0146-6380(00)00061-9.
Yang, J., J. Hatcherian, A. E. Pomerantz, and P. C. Hackley, 2016, New technique for in situ geochemical characterization of dispersed organic matter: Application of infrared (IR) nanoscopy to New Albany Shale (abs.): 2016 Joint Meeting The Society for Organic Petrology–AASP-The Palynological Society–The International Committee for Coal and Organic Petrology, Houston, Texas, September 18–23, 2016, p. 127–128.
AUTHORS
Paul Hackley is a research geologist at the US Geological Survey (USGS) in Reston, Virginia, where he manages the Organic Petrology Laboratory. He holds degrees from Shippensburg University (B.A.), George Washington University (M.Sc.), and George Mason University (Ph.D.). His primary research interests are in organic petrology and its application to fossil fuel assessment.
Mike Lewan is a geochemist and geologist with more than 45 years of experience, which includes employment with Shell Oil Company in their New Orleans offshore exploration and production group, Amoco Production Company at their Tulsa Research Center, and the USGS on their Central Energy Resources Team. He received his Ph.D. from the University of Cincinnati, M.Sc. from Michigan Technological University, and B.Sc. from Northern Illinois University.
ACKNOWLEDGMENTS
Technical reviews of this manuscript at US Geological Survey (USGS) by Paul Lillis, Robert Burruss, and David Houseknecht and editorial comments from Elizabeth Koozmin and Gary Mahon were helpful. Journal reviews by Brian Cardott and an anonymous reviewer and comments from AAPG Editor Barry Katz also improved the manuscript. Mikell Paige (George Mason University) and Ricardo Olea (USGS) assisted with statistical analysis. Peter Warwick, Paul Lillis, Justin Birdwell (USGS), James Donnelly (Bureau of Economic Geology), Matthew Erenpreiss (Ohio Geological Survey), Brian Cardott (Oklahoma Geological Survey), Neil Schovsbo (Geological Survey of Denmark and Greenland), Shannon Daugherty (Kentucky Geological Survey), and Raphael Wüst (Trican) contributed many of the samples  used
used in this study (Table 1). Gregory Baugher (USGS) assisted in the petrographic laboratory. Frank Dulong (USGS) provided x-ray diffraction analyses. Vicky Rocha (Weatherford) and Augusta Warden (USGS) coordinated geochemical analyses. This research was funded by the USGS Energy Resources Program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the United States Government.
in this study (Table 1). Gregory Baugher (USGS) assisted in the petrographic laboratory. Frank Dulong (USGS) provided x-ray diffraction analyses. Vicky Rocha (Weatherford) and Augusta Warden (USGS) coordinated geochemical analyses. This research was funded by the USGS Energy Resources Program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the United States Government.
